NTP Annual Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Report of the Advisory Group to Recommend Priorities for the IARC Monographs During 2020–2024
IARC Monographs on the Identification of Carcinogenic Hazards to Humans Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024 Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024 CONTENTS Introduction ................................................................................................................................... 1 Acetaldehyde (CAS No. 75-07-0) ................................................................................................. 3 Acrolein (CAS No. 107-02-8) ....................................................................................................... 4 Acrylamide (CAS No. 79-06-1) .................................................................................................... 5 Acrylonitrile (CAS No. 107-13-1) ................................................................................................ 6 Aflatoxins (CAS No. 1402-68-2) .................................................................................................. 8 Air pollutants and underlying mechanisms for breast cancer ....................................................... 9 Airborne gram-negative bacterial endotoxins ............................................................................. 10 Alachlor (chloroacetanilide herbicide) (CAS No. 15972-60-8) .................................................. 10 Aluminium (CAS No. 7429-90-5) .............................................................................................. 11 -

A Thesis Entitled Evaluating UVB and UVA Boosting Technologies For
A Thesis entitled Evaluating UVB and UVA Boosting Technologies for Chemical and Physical Sunscreens by An Ngoc Hiep Huynh Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Master of Science Degree in Pharmaceutical Sciences Industrial Pharmacy ___________________________________________ Gabriella Baki, Ph.D., Committee Chair ___________________________________________ Jerry Nesamony, Ph.D., Committee Member ___________________________________________ Matthew W. Liberatore, Ph.D., Committee Member ___________________________________________ Dr. Amanda C. Bryant-Friedrich, Dean College of Graduate Studies The University of Toledo May 2020 Copyright 2020 An Ngoc Hiep Huynh This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of Evaluating UVB and UVA Boosting Technologies for Chemical and Physical Sunscreens by An Ngoc Hiep Huynh Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Master of Science Degree in Pharmaceutical Sciences Industrial Pharmacy The University of Toledo May 2020 There are currently 14 organic and 2 inorganic UV filters approved in the United States. Due to coral reef safety concerns, octinoxate and oxybenzone have been banned in Hawaii, Key West, FL and the US Virgin Islands; and octocrylene is also being studied for its potential impact on coral reef safety, leaving 11 organic UV filters as viable options for sunscreen manufacturers – with limitations on their combination. Since consumers are always looking for sunscreens with high SPF and broad-spectrum protection, the need for UVB and UVA protection boosting technologies is greater than ever. In a preliminary study, about two dozen emollients were scanned for their SPF boosting capability with selected organic UV filters. -

Octinoxate, Octisalate, Avobenzone, Ensulizole, Homosalate
TONYMOLY MAGIC FOOD MANGO MILD SUN BLOCK- octinoxate, octisalate, avobenzone, ensulizole, homosalate cream TONYMOLY CO.,LTD Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies. ---------- ACTIVE INGREDIENT Active ingredients: Ethylhexyl Methoxycinnamate 6.9%, Ethylhexyl Salicylate 4.5%, Butyl Methoxydibenzoylmethane 3.5%, Phenylbenzimidazole Sulfonic Acid 3.5%, Homosalate 3.0% INACTIVE INGREDIENT Inactive ingredients: Water, Butylene Glycol, Alcohol Denat., Octocrylene, Phenethyl Benzoate, Aminomethyl Propanol, Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine, Triceteareth-4 Phosphate, Glycol Stearate, Ammonium Acryloyldimethyltaurate/VP Copolymer, Carbomer, PEG-2 Stearate, Fragrance(Parfum), Phenoxyethanol, Glyceryl Caprylate, Caprylyl Glycol, Mangifera Indica (Mango) Seed Butter, Disodium EDTA, Citrus Limon (Lemon) Fruit Extract, Musa Sapientum (Banana) Fruit Extract, Propylene Glycol, 1,2-Hexanediol, Citrus Aurantium Dulcis (Orange) Fruit Extract, Mangifera Indica (Mango) Fruit Extract, Psidium Guajava Fruit Extract, Citrus Paradisi (Grapefruit) Fruit Extract, Cocos Nucifera (Coconut) Fruit Extract, Actinidia Chinensis (Kiwi) Fruit Extract, Carica Papaya (Papaya) Fruit Extract, Ethylhexylglycerin PURPOSE Purpose: Sunscreen WARNINGS Warnings: For external use only Do not use on damaged or broken skin Stop use and ask a doctor if irritation occurs Keep out of reach of children DESCRIPTION Uses: - helps prevent sunburn - If used as directed with other sun protection measures Directions: decreases the risk of skin cancer and early skin aging caused by the sun Directions: For sunscreen use: - apply liberally 15 minutes before sun exposure - use a water resistant sunscreen if swimming or sweating - reapply at least every 2 hours - Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. -

Hydrolysis of Haloacetonitriles: Linear Free Energy Relationship, Kinetics and Products
Wat. Res. Vol. 33, No. 8, pp. 1938±1948, 1999 # 1999 Elsevier Science Ltd. All rights reserved Printed in Great Britain PII: S0043-1354(98)00361-3 0043-1354/99/$ - see front matter HYDROLYSIS OF HALOACETONITRILES: LINEAR FREE ENERGY RELATIONSHIP, KINETICS AND PRODUCTS VICTOR GLEZER*, BATSHEVA HARRIS, NELLY TAL, BERTA IOSEFZON and OVADIA LEV{*M Division of Environmental Sciences, Fredy and Nadine Herrmann School of Applied Science, The Hebrew University of Jerusalem, 91904, Jerusalem, Israel (First received September 1997; accepted in revised form August 1998) AbstractÐThe hydrolysis rates of mono-, di- and trihaloacetonitriles were studied in aqueous buer sol- utions at dierent pH. The stability of haloacetonitriles decreases and the hydrolysis rate increases with increasing pH and number of halogen atoms in the molecule: The monochloroacetonitriles are the most stable and are also less aected by pH-changes, while the trihaloacetonitriles are the least stable and most sensitive to pH changes. The stability of haloacetonitriles also increases by substitution of chlorine atoms with bromine atoms. The hydrolysis rates in dierent buer solutions follow ®rst order kinetics with a minimum hydrolysis rate at intermediate pH. Thus, haloacetonitriles have to be preserved in weakly acid solutions between sampling and analysis. The corresponding haloacetamides are formed during hydrolysis and in basic solutions they can hydrolyze further to give haloacetic acids. Linear free energy relationship can be used for prediction of degradation of haloacetonitriles -
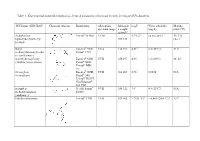
Table 1 - Experimental and Predicted Physical-Chemical Parameters of the Most Recently Investigated UV-Absorbers
Table 1 - Experimental and predicted physical-chemical parameters of the most recently investigated UV-absorbers. INCI name (INN/XAN) Chemical structure Brand name Absorption Molecula LogP Water solubility Melting spectrum range r weight (mg/L) point (°C) (g/mol)4 diethylamino Uvinul® A Plus UVA1 5.7-6.21 <0.01 (20°C) 1 54; 314 hydroxybenzoyl hexyl 397.515 (dec.) 1 benzoate Butyl Eusolex® 9020, UVA 310.393 4.514 2.2 (25°C)4 83.54 methoxydibenzoylmetha Parsol® 1789 ne (avobenzone) 4-methylbenzylidene Eusolex® 6300 UVB 258.397 4.95 1.3 (20°C) 66–68 camphor (enzacamene) Parsol® 5000 Uvinul® MBC 95 Octocrylene Eusolex® OCR, UVB 361.485 6.783 0.00383 N/A (octocrilene) Parsol® 340, Uvinul® N539T, NeoHeliopan® 303 USP isoamyl p- Neo Heliopan® UVB 248.322 3.61 4.9 (25°C)1 N/A methoxycinnamate E1000 (amiloxate) Ethylhexyl triazone Uvinul® T150 UVB 823.092 > 7(20 °C) 6 < 0.001 (20.0 °C) 6 1296 Ethylhexyl Parsol® MCX, UVB 290.403 6.14 0.041 (24 °C and N/A methoxycinnamate Heliopan® New pH 7.1) 4 (octinoxate) Ethylhexyl dimethyl Escalol™ 507 UVB 277.4084 5.774 0.54 (25 °C) 4 N/A PABA (padimate-O) Arlatone 507 Eusolex 6007 benzophenone-3 Eusolex® 4360 UVA2+ UVB 228.247 3.72 3.7 (20°C) 2 62-652 (oxybenzone) bis-ethylhexyloxyphenol Tinosorb® S UVA1+UVB 627.826 12.61 <10-4 80.401 methoxyphenol triazine (bemotrizinol) Phenylbenzimidazole Eusolex® 232 UVA2+ UVB 274.2945 -1.1 (pH 5) > 30% (As sodium N/A sulfonic acid Parsol® HS -2.1 (pH 8)5 or (ensulizole) Neo Heliopan® triethanolammoniu Hydro m salt at 20°C) 5 1 (3) 2 (34) 3 (44) 4 Pubchem 5 SCCP/1056/06 Opinion on phenylbenzimidazole sulfonic acid and its salts 6 BASF safety data sheet Table 2 – In vitro studies for the assessment of skin permeation/penetration of sunscreens. -

Toxicological Review of Chloral Hydrate (CAS No. 302-17-0) (PDF)
EPA/635/R-00/006 TOXICOLOGICAL REVIEW OF CHLORAL HYDRATE (CAS No. 302-17-0) In Support of Summary Information on the Integrated Risk Information System (IRIS) August 2000 U.S. Environmental Protection Agency Washington, DC DISCLAIMER This document has been reviewed in accordance with U.S. Environmental Protection Agency policy and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. Note: This document may undergo revisions in the future. The most up-to-date version will be made available electronically via the IRIS Home Page at http://www.epa.gov/iris. ii CONTENTS—TOXICOLOGICAL REVIEW for CHLORAL HYDRATE (CAS No. 302-17-0) FOREWORD .................................................................v AUTHORS, CONTRIBUTORS, AND REVIEWERS ................................ vi 1. INTRODUCTION ..........................................................1 2. CHEMICAL AND PHYSICAL INFORMATION RELEVANT TO ASSESSMENTS ..... 2 3. TOXICOKINETICS RELEVANT TO ASSESSMENTS ............................3 4. HAZARD IDENTIFICATION ................................................6 4.1. STUDIES IN HUMANS - EPIDEMIOLOGY AND CASE REPORTS .................................................6 4.2. PRECHRONIC AND CHRONIC STUDIES AND CANCER BIOASSAYS IN ANIMALS ................................8 4.2.1. Oral ..........................................................8 4.2.2. Inhalation .....................................................12 4.3. REPRODUCTIVE/DEVELOPMENTAL STUDIES ..........................13 -

Are There Single-Well Hydrogen Bonds in Pyridine-Dichloroacetic Acid Complexes? Charles L
Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2009 Are There Single-Well Hydrogen Bonds in Pyridine-Dichloroacetic Acid Complexes? Charles L. Perrin* and Phaneendrasai Karri Electronic Supplementary Information EXPERIMENTAL DETAILS Materials. All pyridines, dichloroacetic acid, and dichloroacetic anhydride were purchased from Aldrich Chemical Company. Pyridines were stored over activated 4Å molecular sieves under dry N2 for several days prior to use. NMR solvents CD2Cl2 and CDCl3 and 1-mL ampoules of H218O (97.6 atom% 18O) were purchased from Cambridge Isotope Labs. Preparation of Cl2CHCOOH-18O2. Dichloroacetyl chloride (0.5 mL, 5 mmol) was stirred in an ice bath with 0.3 mL H218O (16 mmol) for 1 hr, then warmed to 25ºC and stirred. Incorporation of 18O label was monitored by mass spectrometry. After 48 hr the peaks of mono-18O and di-18O2 acids had nearly equal intensites, and the ratio did not change further. The stirring was stopped and water and HCl were evaporated under reduced pressure (<1 mm Hg) for 24 hr. The extent of labeling was confirmed by the 13C NMR spectrum of a 2:1 mixture with unlabeled dichloroacetic acid in CDCl3, which showed three peaks of nearly the same intensity. Preparation of NMR Samples. Samples were prepared under inert atmosphere using syringe transfer techniques. Pyridine or a substituted pyridine (1.2 mmol) was added to dichloromethane- d2 (1 g). Dichloroacetic acid-18O2 (10 uL of 1:1 mixture with dichloroacetic acid-18O, 0.06 mmol each) and dichloroacetic acid (5 uL, 0.06 mmol) were added to the solution. -

Safety Data Sheet (SDS) OSHA Hazcom Standard 29 CFR 1910.1200(G) and GHS Rev 03
Page 1/12 Safety Data Sheet (SDS) OSHA HazCom Standard 29 CFR 1910.1200(g) and GHS Rev 03. Reviewed on 09/25/18 Issue date 06/11/15 * 1 Identification ꞏ Product identifier ꞏ Trade name: Dichloroacetic Acid ꞏ CAS Number: 79-43-6 ꞏ EC number: 201-207-0 ꞏ Index number: 607-066-00-5 ꞏ Relevant identified uses of the substance or mixture and uses advised against No further relevant information available. ꞏ Product description Dichloroacetic Acid ꞏ Details of the supplier of the safety data sheet ꞏ Manufacturer/Supplier: Dermatologic Lab & Supply, Inc. 608 13th Ave. Council Bluffs, IA USA 51501-6401 Voice: (800) 831-6273 or (712) 323-3269 Fax: (800) 320-9612 or (712) 323-1156 www.delasco.com ꞏ Emergency telephone number: Chemtrec 800-424-9300 * 2 Hazard(s) identification ꞏ Classification of the substance or mixture GHS08 Health hazard Carc. 2 H351 Suspected of causing cancer. Repr. 1A H360 May damage fertility or the unborn child. GHS05 Corrosion Skin Corr. 1A H314Causes severe skin burns and eye damage. GHS09 Environment Aquatic Acute 1H400Very toxic to aquatic life. ꞏ Label elements ꞏ GHS label elements The substance is classified and labeled according to the Globally Harmonized System (GHS). 41.0 Page 2/12 Safety Data Sheet (SDS) OSHA HazCom Standard 29 CFR 1910.1200(g) and GHS Rev 03. Issue date 06/11/15 Reviewed on 09/25/18 Trade name: Dichloroacetic Acid ꞏ Hazard pictograms GHS05 GHS08 GHS09 ꞏ Signal word Danger ꞏ Hazard-determining components of labeling: Dichloroacetic acid ꞏ Hazard statements Causes severe skin burns and eye damage. -

APPENDIX G Acid Dissociation Constants
harxxxxx_App-G.qxd 3/8/10 1:34 PM Page AP11 APPENDIX G Acid Dissociation Constants § ϭ 0.1 M 0 ؍ (Ionic strength ( † ‡ † Name Structure* pKa Ka pKa ϫ Ϫ5 Acetic acid CH3CO2H 4.756 1.75 10 4.56 (ethanoic acid) N ϩ H3 ϫ Ϫ3 Alanine CHCH3 2.344 (CO2H) 4.53 10 2.33 ϫ Ϫ10 9.868 (NH3) 1.36 10 9.71 CO2H ϩ Ϫ5 Aminobenzene NH3 4.601 2.51 ϫ 10 4.64 (aniline) ϪO SNϩ Ϫ4 4-Aminobenzenesulfonic acid 3 H3 3.232 5.86 ϫ 10 3.01 (sulfanilic acid) ϩ NH3 ϫ Ϫ3 2-Aminobenzoic acid 2.08 (CO2H) 8.3 10 2.01 ϫ Ϫ5 (anthranilic acid) 4.96 (NH3) 1.10 10 4.78 CO2H ϩ 2-Aminoethanethiol HSCH2CH2NH3 —— 8.21 (SH) (2-mercaptoethylamine) —— 10.73 (NH3) ϩ ϫ Ϫ10 2-Aminoethanol HOCH2CH2NH3 9.498 3.18 10 9.52 (ethanolamine) O H ϫ Ϫ5 4.70 (NH3) (20°) 2.0 10 4.74 2-Aminophenol Ϫ 9.97 (OH) (20°) 1.05 ϫ 10 10 9.87 ϩ NH3 ϩ ϫ Ϫ10 Ammonia NH4 9.245 5.69 10 9.26 N ϩ H3 N ϩ H2 ϫ Ϫ2 1.823 (CO2H) 1.50 10 2.03 CHCH CH CH NHC ϫ Ϫ9 Arginine 2 2 2 8.991 (NH3) 1.02 10 9.00 NH —— (NH2) —— (12.1) CO2H 2 O Ϫ 2.24 5.8 ϫ 10 3 2.15 Ϫ Arsenic acid HO As OH 6.96 1.10 ϫ 10 7 6.65 Ϫ (hydrogen arsenate) (11.50) 3.2 ϫ 10 12 (11.18) OH ϫ Ϫ10 Arsenious acid As(OH)3 9.29 5.1 10 9.14 (hydrogen arsenite) N ϩ O H3 Asparagine CHCH2CNH2 —— —— 2.16 (CO2H) —— —— 8.73 (NH3) CO2H *Each acid is written in its protonated form. -

FDA Proposes Sunscreen Regulation Changes February 2019
FDA Proposes Sunscreen Regulation Changes February 2019 The U.S. Food and Drug Administration (FDA) regulates sunscreens to ensure they meet safety and eectiveness standards. To improve the quality, safety, and eectiveness of sunscreens, FDA issued a proposed rule that describes updated proposed requirements for sunscreens. Given the recognized public health benets of sunscreen use, Americans should continue to use broad spectrum sunscreen with SPF 15 or higher with other sun protective measures as this important rulemaking eort moves forward. Highlights of FDA’s Proposals Sunscreen active ingredient safety and eectiveness Two ingredients (zinc oxide and titanium dioxide) are proposed to be safe and eective for sunscreen use and two (aminobenzoic acid (PABA) and trolamine salicylate) are 1 proposed as not safe and eective for sunscreen use. FDA proposes that it needs more safety information for the remaining 12 sunscreen ingredients (cinoxate, dioxybenzone, ensulizole, homosalate, meradimate, octinoxate, octisalate, octocrylene, padimate O, sulisobenzone, oxybenzone, avobenzone). New proposed sun protection factor Sunscreen dosage forms (SPF) and broad spectrum Sunscreen sprays, oils, lotions, creams, gels, butters, pastes, ointments, and sticks are requirements 2 proposed as safe and eective. FDA 3 • Raise the maximum proposed labeled SPF proposes that it needs more data for from SPF 50+ to SPF 60+ sunscreen powders. • Require any sunscreen SPF 15 or higher to be broad spectrum • Require for all broad spectrum products SPF 15 and above, as SPF increases, broad spectrum protection increases New proposed label requirements • Include alphabetical listing of active ingredients on the front panel • Require sunscreens with SPF below 15 to include “See Skin Cancer/Skin Aging alert” on the front panel 4 • Require font and placement changes to ensure SPF, broad spectrum, and water resistance statements stand out Sunscreen-insect repellent combination 5 products proposed not safe and eective www.fda.gov. -

Evaluating Analytical Methods for Detecting Unknown Chemicals in Recycled Water
PROJECT NO. 4992 Evaluating Analytical Methods for Detecting Unknown Chemicals in Recycled Water Evaluating Analytical Methods for Detecting Unknown Chemicals in Recycled Water Prepared by: Keith A. Maruya Charles S. Wong Southern California Coastal Water Research Project Authority 2020 The Water Research Foundation (WRF) is a nonprofit (501c3) organization which provides a unified source for One Water research and a strong presence in relationships with partner organizations, government and regulatory agencies, and Congress. The foundation conducts research in all areas of drinking water, wastewater, stormwater, and water reuse. The Water Research Foundation’s research portfolio is valued at over $700 million. The Foundation plays an important role in the translation and dissemination of applied research, technology demonstration, and education, through creation of research‐based educational tools and technology exchange opportunities. WRF serves as a leader and model for collaboration across the water industry and its materials are used to inform policymakers and the public on the science, economic value, and environmental benefits of using and recovering resources found in water, as well as the feasibility of implementing new technologies. For more information, contact: The Water Research Foundation Alexandria, VA Office Denver, CO Office 1199 North Fairfax Street, Suite 900 6666 West Quincy Avenue Alexandria, VA 22314‐1445 Denver, Colorado 80235‐3098 Tel: 571.384.2100 Tel: 303.347.6100 www.waterrf.org [email protected] ©Copyright 2020 by The Water Research Foundation. All rights reserved. Permission to copy must be obtained from The Water Research Foundation. WRF ISBN: 978‐1‐60573‐503‐0 WRF Project Number: 4992 This report was prepared by the organization(s) named below as an account of work sponsored by The Water Research Foundation. -

Les Filtres UV Dans Les Cosmétiques : Une Présence Obligatoire ?
UNIVERSITÉ DE NANTES UFR SCIENCES PHARMACEUTIQUES ET BIOLOGIQUES ____________________________________________________________________________ ANNÉE 2015 N° THÈSE pour le DIPLÔME D’ÉTAT DE DOCTEUR EN PHARMACIE par Anouk POCHAT Présentée et soutenue publiquement le 14 décembre 2015 Les filtres UV dans les cosmétiques : une présence obligatoire ? Président : Mme Laurence Coiffard, Professeur des universités, Laboratoire de Pharmacie industrielle et Cosmétologie Membres du jury : Directeur de thèse : Mme Céline Couteau, Maître de conférences, HDR, Laboratoire de Pharmacie industrielle et Cosmétologie Mme Françoise PEIGNE , Maitre de conférences à la retraite Page 1 Remerciements A mon président de jury, Professeur à la faculté des sciences pharmaceutiques de Nantes J’exprime mes profonds remerciements à Mme Coiffard, pour m’avoir fait l’honneur de présider mon jury de thèse. A mon directeur de thèse, Maître de conférences à la faculté de Pharmacie de Nantes Je remercie Mme Couteau pour m’avoir conseillée et guidée tout au long de mon travail. A Madame Françoise PEIGNE, Docteur en Pharmacie, Je remercie Mme Peigné d’avoir accepté d’assister à ma soutenance. A ma mère, Je te remercie de m’avoir soutenue et encouragée tout au long de mes études. A mon conjoint, Je te remercie pour ta patience, ton écoute et ton soutien. A mes frères, Je vous remercie pour vos encouragements Page 2 I.Introduction Une exposition prolongée aux UVA et aux UVB peut avoir de graves conséquences sur la santé comme, par exemple, la survenue de cancers cutanés (Aubin F., 2001). Les filtres UV permettent d’assurer une protection dans les domaines UVA et/ou UVB. On en trouve dans les produits de protection solaire que le public utilise ponctuellement lors des expositions prolongées au soleil.