Federal Register/Vol. 84, No. 38/Tuesday, February 26, 2019
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Report of the Advisory Group to Recommend Priorities for the IARC Monographs During 2020–2024
IARC Monographs on the Identification of Carcinogenic Hazards to Humans Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024 Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024 CONTENTS Introduction ................................................................................................................................... 1 Acetaldehyde (CAS No. 75-07-0) ................................................................................................. 3 Acrolein (CAS No. 107-02-8) ....................................................................................................... 4 Acrylamide (CAS No. 79-06-1) .................................................................................................... 5 Acrylonitrile (CAS No. 107-13-1) ................................................................................................ 6 Aflatoxins (CAS No. 1402-68-2) .................................................................................................. 8 Air pollutants and underlying mechanisms for breast cancer ....................................................... 9 Airborne gram-negative bacterial endotoxins ............................................................................. 10 Alachlor (chloroacetanilide herbicide) (CAS No. 15972-60-8) .................................................. 10 Aluminium (CAS No. 7429-90-5) .............................................................................................. 11 -

May 23, 2007 Office of Pesticide Programs
May 23, 2007 Office of Pesticide Programs (OPP) Regulatory Public Docket (7502P) Environmental Protection Agency 1200 Pennsylvania Ave., NW Washington, DC 20460-0001 RE: Insect Repellent-Sunscreen Combination Products [EPA-HQ-OPP-2007-0087] Beyond Pesticides appreciates the prudent contemplation of insect repellent-sunscreen combination products EPA proposed in the reregistration eligibility decision (RED) for N,N-diethyl-meta-toluamide (DEET). We also appreciate this opportunity to share our concerns over these products. Beyond Pesticides interest in this issue lies in our effort to restrict pesticide use in a manner that protects public health and the environment, and to advance alternatives that eliminate dependency on toxic chemicals. We oppose the reregistration of all DEET-sunscreen combination products for the following reasons: 1. DEET exposure can result in negative health effects. As the agency notes, the registration of DEET is unusual in that it is one of few residential-use pesticides that is applied directly to the skin. The result is that the public is being exposed to a pesticide that has the ability to cause in lab animals increased fetal loss, bone and skeleton abnormalities in the offspring of rabbits, birth defects in birds, reduction in size of the testes and degeneration, and has produced abnormal sperm with reduced motility. Additionally, the public is directly applying a chemical to their skin that is demonstrated to cross the placenta and move into fetal blood in humans, has the ability to cause mutagenicity and oxidative stress, can decrease sensory and motor skills, causes skin irritation and kills brain cells.1 2. Sunscreen exposure can result in negative health effects. -

A Thesis Entitled Evaluating UVB and UVA Boosting Technologies For
A Thesis entitled Evaluating UVB and UVA Boosting Technologies for Chemical and Physical Sunscreens by An Ngoc Hiep Huynh Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Master of Science Degree in Pharmaceutical Sciences Industrial Pharmacy ___________________________________________ Gabriella Baki, Ph.D., Committee Chair ___________________________________________ Jerry Nesamony, Ph.D., Committee Member ___________________________________________ Matthew W. Liberatore, Ph.D., Committee Member ___________________________________________ Dr. Amanda C. Bryant-Friedrich, Dean College of Graduate Studies The University of Toledo May 2020 Copyright 2020 An Ngoc Hiep Huynh This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of Evaluating UVB and UVA Boosting Technologies for Chemical and Physical Sunscreens by An Ngoc Hiep Huynh Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Master of Science Degree in Pharmaceutical Sciences Industrial Pharmacy The University of Toledo May 2020 There are currently 14 organic and 2 inorganic UV filters approved in the United States. Due to coral reef safety concerns, octinoxate and oxybenzone have been banned in Hawaii, Key West, FL and the US Virgin Islands; and octocrylene is also being studied for its potential impact on coral reef safety, leaving 11 organic UV filters as viable options for sunscreen manufacturers – with limitations on their combination. Since consumers are always looking for sunscreens with high SPF and broad-spectrum protection, the need for UVB and UVA protection boosting technologies is greater than ever. In a preliminary study, about two dozen emollients were scanned for their SPF boosting capability with selected organic UV filters. -

Senate Passage Media Clips Report September 19, 2014
Senate Passage Media Clips Report September 19, 2014 1. The Hill, September 17, 2014, Senate passes bill to improve sunscreen. By Ramsey Cox 2. TIME, September 17, 2014, Senate Passes Bill for Better Sunscreen. By Alexandra Sifferlin 3. The Daily Caller, September 18, 2014, Senate Bill Passes To End FDA Stranglehold On Sunscreen Innovation. By Jonah Bennet 4. Skin, Inc. September 19, 2014, 2014 Sunscreen Innovation Act Unanimously Passes. 5. Inside Health (Subscription), September 18, 2014, Sunscreen Legislation Backers Seek Swift Enactment After Senate Clears Bill. By Stephanie Beasley 6. Politico Pulse (Subscription), September 18, 2014, Sunscreen bill heads to the House now. 7. Politico Pulse (Subscription), September 18, 2014, HELP approve sunscreen bill. 8. Office of Senator Kay Hagan, September 19, 2014, Bipartisan Sunscreen Innovation Act Cosponsored by Hagan Passes the Senate. 9. Northwest Georgia News, September 18, 2014, Isakson applauds Senate passage of bipartisan bill to help prevent skin cancer. 10. Primer{TBR}, September 18, 2014, Senate Passes Sunscreen Innovation Act to Improve Sunscreen. 11. Smithsonian, September 18, 2014, The US Is Trying to Expedite Sunscreen Innovation. By Rachel Nuwer 12. Cosmetics & Toiletries, September 18, 2014, Sunscreen Innovation Act Passes: What Does it Mean? By Rachel Grahenhofer 13. Office of Senator Mitch McConnell (Press Release), September 18, 2014, McConnell- Sponsored Sunscreen Innovation Act Passes Senate. 14. MarketPlace, September 18, 2014, Your sunscreen is way out of date. By Nancy Marshall-Genzer 37#32748001_v1 15. Earthy Essence, September 18, 2014, 2014 Sunscreen Innovation Act Unanimously Passes. 16. Melanoma Research Alliance, September 17, 2014, MRA Applauds Senate Passage of the Sunscreen Innovation Act. -

GAO-18-61, SUNSCREEN: FDA Reviewed Applications For
United States Government Accountability Office Report to Congressional Committees November 2017 SUNSCREEN FDA Reviewed Applications for Additional Active Ingredients and Determined More Data Needed GAO-18-61 November 2017 SUNSCREEN FDA Reviewed Applications for Additional Active Ingredients and Determined More Data Needed Highlights of GAO-18-61, a report to congressional committees Why GAO Did This Study What GAO Found Using sunscreen as directed with other The Food and Drug Administration (FDA), within the Department of Health and sun protective measures may help Human Services, implemented requirements for reviewing applications for reduce the risk of skin cancer—the sunscreen active ingredients within time frames set by the Sunscreen Innovation most common form of cancer in the Act, which was enacted in November 2014. For example, the agency issued a United States. In the United States, guidance document on safety and effectiveness testing in November 2016. sunscreen is considered an over-the- counter drug, which is a drug available As of August 2017, all applications for sunscreen active ingredients remain to consumers without a prescription. pending after the agency determined more safety and effectiveness data are Some sunscreen active ingredients not needed. By February 2015, FDA completed its initial review of the safety and currently marketed in the United States effectiveness data for each of the eight pending applications, as required by the have been available in products in act. FDA concluded that additional data are needed to determine that the other countries for more than a ingredients are generally recognized as safe and effective (GRASE), which is decade. Companies that manufacture needed so that products using the ingredients can subsequently be marketed in some of these ingredients have sought the United States without FDA’s premarket approval. -

Toxic Effects
Chapter 8 Toxic effects Toxic and other adverse effects studies of tens or hundreds of patients spectrum to which the skin is exposed of sunscreens (Thune, 1984; English et al., 1987; (Gasparro et al., 1998). Since UVB is the In order for a sunscreen to have a toxic Lenique et al., 1992; Szczurko et al., primary stimulus for adaptation of the effect on living tissues, it must penetrate 1994; Trevisi et al., 1994; Gonçalo et al., skin to sunlight, less adaptation might be the skin. There is some evidence that 1995; Ang et al., 1998) and reviews expected to develop in individuals who this can occur (see p. 63 et seq.). (Dromgoole & Maibach, 1990; Gonzalez use sunscreens regularly. The adaptive & Gonzalez, 1996; Schauder & Ippen, responses include thickening of the epi- Human studies 1997). In the past, PABA and its esters dermis and transfer of melanin-contain- No published studies of toxic effects in were the most commonly reported ing granules to keratinocytes (tanning) humans were available to the Working contact and photoallergens in sun- (Fig. 44), which reduces the trans- Group. screens (Funk et ai., 1997), and this find- parency of the skin to UVA and UVB ing contributed to a reduction in their use (Fusaro et al., 1966; Olson et al., 1973). Contact sensitivity in sunscreens. The contact or photocon- Several reports showed that UVR- There are numerous reports of cases of tact allergen in sunscreens most induced injury, such as dermal connec- allergic reactions and photoreactivity to frequently cited today is benzophenone- tive tissue damage and sunburn cell for- sunscreens, but the prevalence of this 3, followed by dibenzoyl methanes. -

Octinoxate, Octisalate, Avobenzone, Ensulizole, Homosalate
TONYMOLY MAGIC FOOD MANGO MILD SUN BLOCK- octinoxate, octisalate, avobenzone, ensulizole, homosalate cream TONYMOLY CO.,LTD Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies. ---------- ACTIVE INGREDIENT Active ingredients: Ethylhexyl Methoxycinnamate 6.9%, Ethylhexyl Salicylate 4.5%, Butyl Methoxydibenzoylmethane 3.5%, Phenylbenzimidazole Sulfonic Acid 3.5%, Homosalate 3.0% INACTIVE INGREDIENT Inactive ingredients: Water, Butylene Glycol, Alcohol Denat., Octocrylene, Phenethyl Benzoate, Aminomethyl Propanol, Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine, Triceteareth-4 Phosphate, Glycol Stearate, Ammonium Acryloyldimethyltaurate/VP Copolymer, Carbomer, PEG-2 Stearate, Fragrance(Parfum), Phenoxyethanol, Glyceryl Caprylate, Caprylyl Glycol, Mangifera Indica (Mango) Seed Butter, Disodium EDTA, Citrus Limon (Lemon) Fruit Extract, Musa Sapientum (Banana) Fruit Extract, Propylene Glycol, 1,2-Hexanediol, Citrus Aurantium Dulcis (Orange) Fruit Extract, Mangifera Indica (Mango) Fruit Extract, Psidium Guajava Fruit Extract, Citrus Paradisi (Grapefruit) Fruit Extract, Cocos Nucifera (Coconut) Fruit Extract, Actinidia Chinensis (Kiwi) Fruit Extract, Carica Papaya (Papaya) Fruit Extract, Ethylhexylglycerin PURPOSE Purpose: Sunscreen WARNINGS Warnings: For external use only Do not use on damaged or broken skin Stop use and ask a doctor if irritation occurs Keep out of reach of children DESCRIPTION Uses: - helps prevent sunburn - If used as directed with other sun protection measures Directions: decreases the risk of skin cancer and early skin aging caused by the sun Directions: For sunscreen use: - apply liberally 15 minutes before sun exposure - use a water resistant sunscreen if swimming or sweating - reapply at least every 2 hours - Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. -
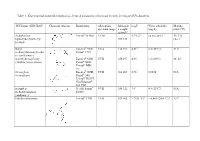
Table 1 - Experimental and Predicted Physical-Chemical Parameters of the Most Recently Investigated UV-Absorbers
Table 1 - Experimental and predicted physical-chemical parameters of the most recently investigated UV-absorbers. INCI name (INN/XAN) Chemical structure Brand name Absorption Molecula LogP Water solubility Melting spectrum range r weight (mg/L) point (°C) (g/mol)4 diethylamino Uvinul® A Plus UVA1 5.7-6.21 <0.01 (20°C) 1 54; 314 hydroxybenzoyl hexyl 397.515 (dec.) 1 benzoate Butyl Eusolex® 9020, UVA 310.393 4.514 2.2 (25°C)4 83.54 methoxydibenzoylmetha Parsol® 1789 ne (avobenzone) 4-methylbenzylidene Eusolex® 6300 UVB 258.397 4.95 1.3 (20°C) 66–68 camphor (enzacamene) Parsol® 5000 Uvinul® MBC 95 Octocrylene Eusolex® OCR, UVB 361.485 6.783 0.00383 N/A (octocrilene) Parsol® 340, Uvinul® N539T, NeoHeliopan® 303 USP isoamyl p- Neo Heliopan® UVB 248.322 3.61 4.9 (25°C)1 N/A methoxycinnamate E1000 (amiloxate) Ethylhexyl triazone Uvinul® T150 UVB 823.092 > 7(20 °C) 6 < 0.001 (20.0 °C) 6 1296 Ethylhexyl Parsol® MCX, UVB 290.403 6.14 0.041 (24 °C and N/A methoxycinnamate Heliopan® New pH 7.1) 4 (octinoxate) Ethylhexyl dimethyl Escalol™ 507 UVB 277.4084 5.774 0.54 (25 °C) 4 N/A PABA (padimate-O) Arlatone 507 Eusolex 6007 benzophenone-3 Eusolex® 4360 UVA2+ UVB 228.247 3.72 3.7 (20°C) 2 62-652 (oxybenzone) bis-ethylhexyloxyphenol Tinosorb® S UVA1+UVB 627.826 12.61 <10-4 80.401 methoxyphenol triazine (bemotrizinol) Phenylbenzimidazole Eusolex® 232 UVA2+ UVB 274.2945 -1.1 (pH 5) > 30% (As sodium N/A sulfonic acid Parsol® HS -2.1 (pH 8)5 or (ensulizole) Neo Heliopan® triethanolammoniu Hydro m salt at 20°C) 5 1 (3) 2 (34) 3 (44) 4 Pubchem 5 SCCP/1056/06 Opinion on phenylbenzimidazole sulfonic acid and its salts 6 BASF safety data sheet Table 2 – In vitro studies for the assessment of skin permeation/penetration of sunscreens. -

Sun Protection, Sunscreens and Vitamin D
SunSun protection,protection, sunscreenssunscreens andand VitaminVitamin DD GPGP NationalNational ConferenceConference RotoruaRotorua EnergyEnergy EventsEvents CentreCentre JuneJune 20092009 Dr. Louise Reiche Dermatologist New Zealand Dermatological Society Incorporated MelanomaMelanoma SkinSkin cancercancer andand sunlightsunlight Exposure to UVR causes > 90% of skin cancers Skin cancer is commonest cancer in NZ >50,000 new cases per year ~300 deaths per year ~$33.4 NZ million per year International Agency for Research on Cancer. IARC Monographs on the evaluation of carcinogenic risks to humans. Solar ultraviolet radiation. Lyon: International Agency for Research on Cancer, 1992. Armstrong BK. How sun exposure causes skin cancer. In: Hill D, Elwood JM, English DR, Eds. Prevention of Skin Cancer. Dordrecht: Kluwer Academic Publishers, 2004. O’Dea D. The Costs of Skin Cancer to New Zealand. Wellington: Cancer Society of New Zealand, 2000. New Zealand Health Information Service. Cancer, New Registrations and Deaths. Wellington: New Zealand Health Information Service, 2004. MelanomaMelanoma 1842 new cases in 2002 328 directly attributable to severe sunburn (Sneyd and Cox 2006) Authors recommended, “to reduce burden of melanoma in NZ, need to prevent excessive sun exposure and (facilitate) early diagnosis” Whilst cancer overall is rare in adolescence, melanoma was commonest cancer MelanomaMelanoma NZ incidence and death rate among world highest 56.2/100,000 in European population of Auckland highest reported worldwide men -

FDA Proposes Sunscreen Regulation Changes February 2019
FDA Proposes Sunscreen Regulation Changes February 2019 The U.S. Food and Drug Administration (FDA) regulates sunscreens to ensure they meet safety and eectiveness standards. To improve the quality, safety, and eectiveness of sunscreens, FDA issued a proposed rule that describes updated proposed requirements for sunscreens. Given the recognized public health benets of sunscreen use, Americans should continue to use broad spectrum sunscreen with SPF 15 or higher with other sun protective measures as this important rulemaking eort moves forward. Highlights of FDA’s Proposals Sunscreen active ingredient safety and eectiveness Two ingredients (zinc oxide and titanium dioxide) are proposed to be safe and eective for sunscreen use and two (aminobenzoic acid (PABA) and trolamine salicylate) are 1 proposed as not safe and eective for sunscreen use. FDA proposes that it needs more safety information for the remaining 12 sunscreen ingredients (cinoxate, dioxybenzone, ensulizole, homosalate, meradimate, octinoxate, octisalate, octocrylene, padimate O, sulisobenzone, oxybenzone, avobenzone). New proposed sun protection factor Sunscreen dosage forms (SPF) and broad spectrum Sunscreen sprays, oils, lotions, creams, gels, butters, pastes, ointments, and sticks are requirements 2 proposed as safe and eective. FDA 3 • Raise the maximum proposed labeled SPF proposes that it needs more data for from SPF 50+ to SPF 60+ sunscreen powders. • Require any sunscreen SPF 15 or higher to be broad spectrum • Require for all broad spectrum products SPF 15 and above, as SPF increases, broad spectrum protection increases New proposed label requirements • Include alphabetical listing of active ingredients on the front panel • Require sunscreens with SPF below 15 to include “See Skin Cancer/Skin Aging alert” on the front panel 4 • Require font and placement changes to ensure SPF, broad spectrum, and water resistance statements stand out Sunscreen-insect repellent combination 5 products proposed not safe and eective www.fda.gov. -

New Sunscreen Approval — Why So Complicated?
Watch Pages New Sunscreen Approval — Why So Complicated? Topically-applied sunscreen product use is a billion-dollar These popular sunscreens have been submitted to the industry. The use of these products over the last 40 US Food and Drug Administration (FDA) over the last 15 years has changed dramatically from a “niche” market of years for approval. The FDA has approved none of these fair-skinned individuals who used small amounts on an products — which are regulated as drugs in the US — irregular basis to the current market of a large swath of despite years of use in Europe, Asia, or Latin America, the adult and paediatric population, fair-skinned or not, where they are regulated as cosmetics. The last over-the- over larger areas of their bodies on a much more regular counter (OTC) sunscreen approved by the FDA was in the basis — in some cases, daily. late 1990s. That said, the agency has not rejected these products either. Benefits of sunscreen use (SPF 15+) are widely accepted: sunburn prevention, skin-aging reduction, and, Instead, the products are in a nebulous regulatory of particular importance, skin cancer risk reduction. The area due to their classification and lack of criteria to benefits and increased usage have resulted in demand determine the type of clinical data needed to prove they for and development of a wide array of new sunscreen are “generally recognised as safe and effective” (GRASE), products over the last 20 years. These modern products an essential designation needed for OTC approval. This offer enhanced protection against the ultraviolet A (UVA) designation is usually granted, and the drug thereby rays that play a larger part in skin cancer than was once regulated, under what is called the OTC drug monograph believed, as well as the traditional protection against process. -

Kroger Co Disclaimer: Most OTC Drugs Are Not Reviewed and Approved by FDA, However They May Be Marketed If They Comply with Applicable Regulations and Policies
SUNSCREEN- avobenzone, homosalate, octisalate, octocrylene, oxybenzone lotion The Kroger Co Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies. ---------- Kroger 711 Active Ingredients Avobenzone 3% Homosalate 13% Octisalate 5% Octocrylene 7% Oxybenzone 4% Purpose Sunscreen Use helps prevent sunburn if used as directed with other sun protections measures (see Directions), decreases the rist of skin cancer and early skin aging caused by the sun Warnings For external use only Do not use on damaged or broken skin When using this product keep out of eyes. Rinse with water to remove Stop use and ask a doctor if rash occurs Keep out of reach of children if swallowed, get medical help or contact a Poison Control Center right away Directions apply liberally 15 minutes before sun exposure reapply: after 80 minutes of swimming or sweating immediately after towel drying at least every 2 hours Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk use a reqularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 a.m. - 2 p.m. wear long-sleeved shirts, pants, hats and sunglasses children under 6 months of age: Ask a doctor Other Information Protect the product from excessive heat and direct sun Inactive ingredients water, sorbitol, triethanolamine, VP/eicosene copolymer, stearic acid, sorbitan isostearate, aluminum starch octenylsuccinate, benzyl alcohol, dimethicone, tocopheryl, chlorphenesin, polyglyceryl-3 distearate, fragrance, carbomer, disodium EDTA Questions or comments? 1-800-632-6900 Adverse Reaction DISTRIBUTED BY THE KROGER CO.