A Guide to United States Cosmetic Products Compliance Requirements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diseño De Un Envase Dosificador De Desodorante Femenino Y Masculino, Para La Empresa Interplast S.A
DISEÑO DE UN ENVASE DOSIFICADOR DE DESODORANTE FEMENINO Y MASCULINO, PARA LA EMPRESA INTERPLAST S.A. ALEJANDRO CARVAJAL MONSALVE Trabajo de grado presentado como requisito parcial para optar al título de Ingeniero de Diseño de Producto Asesor: CAROLINA ALZATE ALVAREZ Ingeniera de Diseño de Producto MEDELLÍN UNIVERSIDAD EAFIT DEPARTAMENTO DE INGENIERÍA DE DISEÑO DE PRODUCTO 2009 Nota de Aceptación _________________________________________________ _________________________________________________ _________________________________________________ _________________________________________________ Presidente de Jurado ________________________________________________ Jurado ________________________________________________ Jurado ________________________________________________ Medellín, 13 de Octubre de 2009 2 A mi madre, familiares y amigos que me apoyaron en toda la elaboración del proyecto. Y por amor a la memoria llevo sobre mi cara la cara de mi padre. YEHUDA AMIJAI 3 AGRADECIMIENTOS Internacional de Plásticos S.A. por el apoyo brindado durante todo el proyecto. Carolina Álzate Álvarez, asesora del proyecto de grado, por su acompañamiento, dedicación y asesoría. Jorge Hernán Gómez, Jefe Departamento Técnico de Interplast S.A., por su cooperación para realizar el proyecto. Ana Cecilia Restrepo, Jefe Aseguramiento de la Calidad de Interplast S.A., por su asesoría y ayuda brindada. Luz Mery Rámirez, tecnóloga de producción Interplast S.A, por su ayuda en el análisis de los envases de la compañía. Familiares, compañeros de trabajo y amigos que de alguna forma ofrecieron su colaboración para llevar a cabo este proyecto con éxito. 4 CONTENIDO Pág. LISTA DE TABLAS 8 LISTA DE FIGURAS 10 LISTA DE ANEXOS 15 GLOSARIO 16 RESUMEN 18 INTRODUCCIÓN 19 ANTECEDENTES 21 JUSTIFICACIÓN 24 OBJETIVOS 26 OBJETIVO GENERAL 26 OBJETIVOS ESPECIFICOS 26 ALCANCE 27 METODOLOGÍA 28 MARCO TEORICO 29 2.1 INVESTIGACIÓN CONTEXTO INTERNO 30 2.1.1 INVESTIGACIÓN DE LA COMPAÑÍA 30 2.1.2 PRODUCTOS DE INTERPLAST S.A. -

A Thesis Entitled Evaluating UVB and UVA Boosting Technologies For
A Thesis entitled Evaluating UVB and UVA Boosting Technologies for Chemical and Physical Sunscreens by An Ngoc Hiep Huynh Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Master of Science Degree in Pharmaceutical Sciences Industrial Pharmacy ___________________________________________ Gabriella Baki, Ph.D., Committee Chair ___________________________________________ Jerry Nesamony, Ph.D., Committee Member ___________________________________________ Matthew W. Liberatore, Ph.D., Committee Member ___________________________________________ Dr. Amanda C. Bryant-Friedrich, Dean College of Graduate Studies The University of Toledo May 2020 Copyright 2020 An Ngoc Hiep Huynh This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of Evaluating UVB and UVA Boosting Technologies for Chemical and Physical Sunscreens by An Ngoc Hiep Huynh Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Master of Science Degree in Pharmaceutical Sciences Industrial Pharmacy The University of Toledo May 2020 There are currently 14 organic and 2 inorganic UV filters approved in the United States. Due to coral reef safety concerns, octinoxate and oxybenzone have been banned in Hawaii, Key West, FL and the US Virgin Islands; and octocrylene is also being studied for its potential impact on coral reef safety, leaving 11 organic UV filters as viable options for sunscreen manufacturers – with limitations on their combination. Since consumers are always looking for sunscreens with high SPF and broad-spectrum protection, the need for UVB and UVA protection boosting technologies is greater than ever. In a preliminary study, about two dozen emollients were scanned for their SPF boosting capability with selected organic UV filters. -

The Beauty Industry's Influence on Women in Society
University of New Hampshire University of New Hampshire Scholars' Repository Honors Theses and Capstones Student Scholarship Fall 2012 The Beauty Industry's Influence on omenW in Society Ann Marie Britton University of New Hampshire - Main Campus Follow this and additional works at: https://scholars.unh.edu/honors Part of the Fashion Business Commons, and the Personality and Social Contexts Commons Recommended Citation Britton, Ann Marie, "The Beauty Industry's Influence on omenW in Society" (2012). Honors Theses and Capstones. 86. https://scholars.unh.edu/honors/86 This Senior Honors Thesis is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Honors Theses and Capstones by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. RUNNING HEAD: THE BEAUTY INDUSTRY’S INFLUENCE ON WOMEN 1 HONORS THESIS The Beauty Industry’s Influence on Women in Society By Ann Marie Britton Fall Semester, 2012 Faculty Sponsor: Bruce E. Pfeiffer, Ph.D. THE BEAUTY INDUSTRY’S INFLUENCE ON WOMEN 2 Abstract There has been a significant amount of research done on the effect that advertising in the fashion and beauty industry has on women. By creating advertisements with unrealistic images of beauty, it has resulted in anxiety, low self-esteem, and low self-confidence in many women. Most of these negative emotions stems from unhappiness among body and appearance. Less research has been performed relating to cosmetics and how this can have an influence on women, and how women can use cosmetics to manipulate their appearance. -

BD Novdec2013 Safirhs.Indd
www.bulk-distributor.com BULKEst. 1990 DISTRIBUTORNovember/December 2013 Your single information source for bulk and semi-bulk logistics 5BOL$POUBJOFSTt'MFYJUBOLTt*#$Tt%SVNTt'*#$Tt#VML-JOFSTt3PBE5BOLFSTt-PBEJOH#BHHJOHt#VML-PHJTUJDTt$MFBOJOH3FQBJS%FQPUTt$PNQPOFOUT IN THIS ISSUE French government forced to Container Packing 2 Shipper 3 suspend écotaxe FIBCs 4 he French government has been forced Tonce again to postpone the IBCs 8 implementation of a tax on heavy good vehicles (HGVs), known as the ‘écotaxe’. Depot Services 10 After previous delays due to ‘technical issues’ the écotaxe was scheduled to be introduced on 1 Tank Containers 13 January 2014. However, violent protests by hauliers, especially ANNUAL REVIEW: 17 in the Brittany region, provoked Prime Minister Flexitanks & Bulk Liners Jean-Marc Ayrault to delay its introduction for an indefinite period. He emphasised the scheme was Dry Bulk Handling 23 not being abandoned but “time was required to make adjustments to it”. Logistics 24 Ecotaxe is supposed to apply to all goods transport vehicles over 3.5 tonnes using the Terminals & Storage 26 15,000km of the French national road network (N roads). Motorway users – lorries and cars - already Managing Editor pay tolls through the péage system. Neil Madden The tax is calculated on a kilometric rate that France’s main road haulage federations have been saying for months that the implementation date was unrealistic [email protected] varies according to the vehicle category and can be modulated according to the vehicle’s pollution record the information needed to define the France’s main road haulage federations have Tel: +33 (0)3 88 60 30 68 level (EURO class). -

Toxic Effects
Chapter 8 Toxic effects Toxic and other adverse effects studies of tens or hundreds of patients spectrum to which the skin is exposed of sunscreens (Thune, 1984; English et al., 1987; (Gasparro et al., 1998). Since UVB is the In order for a sunscreen to have a toxic Lenique et al., 1992; Szczurko et al., primary stimulus for adaptation of the effect on living tissues, it must penetrate 1994; Trevisi et al., 1994; Gonçalo et al., skin to sunlight, less adaptation might be the skin. There is some evidence that 1995; Ang et al., 1998) and reviews expected to develop in individuals who this can occur (see p. 63 et seq.). (Dromgoole & Maibach, 1990; Gonzalez use sunscreens regularly. The adaptive & Gonzalez, 1996; Schauder & Ippen, responses include thickening of the epi- Human studies 1997). In the past, PABA and its esters dermis and transfer of melanin-contain- No published studies of toxic effects in were the most commonly reported ing granules to keratinocytes (tanning) humans were available to the Working contact and photoallergens in sun- (Fig. 44), which reduces the trans- Group. screens (Funk et ai., 1997), and this find- parency of the skin to UVA and UVB ing contributed to a reduction in their use (Fusaro et al., 1966; Olson et al., 1973). Contact sensitivity in sunscreens. The contact or photocon- Several reports showed that UVR- There are numerous reports of cases of tact allergen in sunscreens most induced injury, such as dermal connec- allergic reactions and photoreactivity to frequently cited today is benzophenone- tive tissue damage and sunburn cell for- sunscreens, but the prevalence of this 3, followed by dibenzoyl methanes. -

Annual Report 2018 Contents 1St 86,000 Cosmetics Group Employees Prospects Worldwide(1) 02 Prospects by Jean-Paul Agon, Chairman & CEO
Annual Report 2018 Contents 1st 86,000 cosmetics group employees Prospects worldwide(1) 02 Prospects by Jean-Paul Agon, Chairman & CEO Strategy 36 150 brands countries 06 Governance · The Board of Directors · The Executive Committee 10 Quality 12 Ethics 26.9 505 14 Responsibility · “Sharing Beauty With All” billion euros patents registered · Citizen Day of sales(2) in 2018 · The L’Oréal Corporate Foundation 18 Human Relations Performance Commitments for 2 4.92 1 Cosmetics Market 24 L’Oréal in figures billion euros in 2020 28 Worldwide advances operating profit “Sharing Beauty With All” 31 Strategic themes Brands 33 Brands overview (1) Source: WWD, Beauty Top 100,May 2018. (2) At 31 December 2018. 34 Consumer Products 38 L’Oréal Luxe 42 Professional Products More exclusive content 46 Active Cosmetics on the digital version Expertise lorealannualreport2018.com 52 Research & Innovation 54 Operations Discover and filter 56 Digital the Annual Report content 58 Administration and Finance Discover the strategic themes of the Annual Report. Use them to filter content and personalise your navigation to find content that matches your interests. The digital version also features more exclusive content, articles, infographics and many videos. Our mission Beauty for All Offering all women and men worldwide the best of cosmetics in terms of quality, efficacy and safety to satisfy all their beauty needs and desires, in their infinite diversity. Our strategy Universalisation L’Oréal has chosen a unique strategy: Universalisation. It means globalisation that captures, understands and respects differences. Differences in desires, needs and traditions. To offer tailor-made beauty, and meet the aspirations of consumers in every part of the world. -

Plastic & Synthetic Film Recycling Guide
Plastic & Synthetic Film Recycling Guide The resin identification coding system was introduced in 1988 to meet the need for a nationwide, uniform system of identifying plastics for both manufacturers and recyclers. This guide identifies the seven types of recyclable plastics to help you distinguish among the different plastics in their various forms. Marked with the appropriate symbol, plastic and film products can be properly recycled and used again. GPA strives to bring you a variety of printable films and synthetics that offer you sustainable choices for every application. Code GPA Products Name Properties Applications/Uses • Ultra Film® Clear Polyester Polyethylene Clarity, strength, toughness, barrier Menu boards, water and soft drink bottles, • InCycle™ White Expanded PET Terephthalate to gas and moisture, resistance to membrane switches, overlays, inserts, and • Ultra Digital® Clear Polyester Transparency heat. The easiest and most common many injection molded consumer product Film Common Name: plastic that is recycled. containers. Polyester PETE • Ultra Film® 100% Recycled White High-Density Stiffness, strength, toughness, Semi-permanent signage, interior/exterior Polyethylene (35% post-consumer waste Polyethylene resistance to chemicals and signage, tags, labels, cosmetic packaging, from milk jugs) moisture, permeability to gas, ease yogurt containers and margarine tubs, of processing and ease of forming. grocery and retail bags. HDPE Like #1, widely accepted. • Ultra Film® White & Clear Rigid Vinyl Polyvinyl Chloride Versatility, clarity, ease of blending, Indoor and retail P.O.P signage, shelf talkers, • Ultra Digital® White Rigid Vinyl strength, toughness, resistance to aisle violators, packaging, book covers, Common Name: grease, oil and chemicals. decals, loose-leaf binders, counter mats, Vinyl mouse pads, packaging, floor tiles and mats, cassette trays, clear food and PVC or V non-food packaging. -

Octinoxate, Octisalate, Avobenzone, Ensulizole, Homosalate
TONYMOLY MAGIC FOOD MANGO MILD SUN BLOCK- octinoxate, octisalate, avobenzone, ensulizole, homosalate cream TONYMOLY CO.,LTD Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies. ---------- ACTIVE INGREDIENT Active ingredients: Ethylhexyl Methoxycinnamate 6.9%, Ethylhexyl Salicylate 4.5%, Butyl Methoxydibenzoylmethane 3.5%, Phenylbenzimidazole Sulfonic Acid 3.5%, Homosalate 3.0% INACTIVE INGREDIENT Inactive ingredients: Water, Butylene Glycol, Alcohol Denat., Octocrylene, Phenethyl Benzoate, Aminomethyl Propanol, Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine, Triceteareth-4 Phosphate, Glycol Stearate, Ammonium Acryloyldimethyltaurate/VP Copolymer, Carbomer, PEG-2 Stearate, Fragrance(Parfum), Phenoxyethanol, Glyceryl Caprylate, Caprylyl Glycol, Mangifera Indica (Mango) Seed Butter, Disodium EDTA, Citrus Limon (Lemon) Fruit Extract, Musa Sapientum (Banana) Fruit Extract, Propylene Glycol, 1,2-Hexanediol, Citrus Aurantium Dulcis (Orange) Fruit Extract, Mangifera Indica (Mango) Fruit Extract, Psidium Guajava Fruit Extract, Citrus Paradisi (Grapefruit) Fruit Extract, Cocos Nucifera (Coconut) Fruit Extract, Actinidia Chinensis (Kiwi) Fruit Extract, Carica Papaya (Papaya) Fruit Extract, Ethylhexylglycerin PURPOSE Purpose: Sunscreen WARNINGS Warnings: For external use only Do not use on damaged or broken skin Stop use and ask a doctor if irritation occurs Keep out of reach of children DESCRIPTION Uses: - helps prevent sunburn - If used as directed with other sun protection measures Directions: decreases the risk of skin cancer and early skin aging caused by the sun Directions: For sunscreen use: - apply liberally 15 minutes before sun exposure - use a water resistant sunscreen if swimming or sweating - reapply at least every 2 hours - Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. -
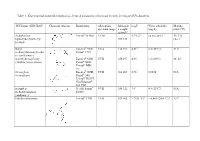
Table 1 - Experimental and Predicted Physical-Chemical Parameters of the Most Recently Investigated UV-Absorbers
Table 1 - Experimental and predicted physical-chemical parameters of the most recently investigated UV-absorbers. INCI name (INN/XAN) Chemical structure Brand name Absorption Molecula LogP Water solubility Melting spectrum range r weight (mg/L) point (°C) (g/mol)4 diethylamino Uvinul® A Plus UVA1 5.7-6.21 <0.01 (20°C) 1 54; 314 hydroxybenzoyl hexyl 397.515 (dec.) 1 benzoate Butyl Eusolex® 9020, UVA 310.393 4.514 2.2 (25°C)4 83.54 methoxydibenzoylmetha Parsol® 1789 ne (avobenzone) 4-methylbenzylidene Eusolex® 6300 UVB 258.397 4.95 1.3 (20°C) 66–68 camphor (enzacamene) Parsol® 5000 Uvinul® MBC 95 Octocrylene Eusolex® OCR, UVB 361.485 6.783 0.00383 N/A (octocrilene) Parsol® 340, Uvinul® N539T, NeoHeliopan® 303 USP isoamyl p- Neo Heliopan® UVB 248.322 3.61 4.9 (25°C)1 N/A methoxycinnamate E1000 (amiloxate) Ethylhexyl triazone Uvinul® T150 UVB 823.092 > 7(20 °C) 6 < 0.001 (20.0 °C) 6 1296 Ethylhexyl Parsol® MCX, UVB 290.403 6.14 0.041 (24 °C and N/A methoxycinnamate Heliopan® New pH 7.1) 4 (octinoxate) Ethylhexyl dimethyl Escalol™ 507 UVB 277.4084 5.774 0.54 (25 °C) 4 N/A PABA (padimate-O) Arlatone 507 Eusolex 6007 benzophenone-3 Eusolex® 4360 UVA2+ UVB 228.247 3.72 3.7 (20°C) 2 62-652 (oxybenzone) bis-ethylhexyloxyphenol Tinosorb® S UVA1+UVB 627.826 12.61 <10-4 80.401 methoxyphenol triazine (bemotrizinol) Phenylbenzimidazole Eusolex® 232 UVA2+ UVB 274.2945 -1.1 (pH 5) > 30% (As sodium N/A sulfonic acid Parsol® HS -2.1 (pH 8)5 or (ensulizole) Neo Heliopan® triethanolammoniu Hydro m salt at 20°C) 5 1 (3) 2 (34) 3 (44) 4 Pubchem 5 SCCP/1056/06 Opinion on phenylbenzimidazole sulfonic acid and its salts 6 BASF safety data sheet Table 2 – In vitro studies for the assessment of skin permeation/penetration of sunscreens. -

Sun Protection, Sunscreens and Vitamin D
SunSun protection,protection, sunscreenssunscreens andand VitaminVitamin DD GPGP NationalNational ConferenceConference RotoruaRotorua EnergyEnergy EventsEvents CentreCentre JuneJune 20092009 Dr. Louise Reiche Dermatologist New Zealand Dermatological Society Incorporated MelanomaMelanoma SkinSkin cancercancer andand sunlightsunlight Exposure to UVR causes > 90% of skin cancers Skin cancer is commonest cancer in NZ >50,000 new cases per year ~300 deaths per year ~$33.4 NZ million per year International Agency for Research on Cancer. IARC Monographs on the evaluation of carcinogenic risks to humans. Solar ultraviolet radiation. Lyon: International Agency for Research on Cancer, 1992. Armstrong BK. How sun exposure causes skin cancer. In: Hill D, Elwood JM, English DR, Eds. Prevention of Skin Cancer. Dordrecht: Kluwer Academic Publishers, 2004. O’Dea D. The Costs of Skin Cancer to New Zealand. Wellington: Cancer Society of New Zealand, 2000. New Zealand Health Information Service. Cancer, New Registrations and Deaths. Wellington: New Zealand Health Information Service, 2004. MelanomaMelanoma 1842 new cases in 2002 328 directly attributable to severe sunburn (Sneyd and Cox 2006) Authors recommended, “to reduce burden of melanoma in NZ, need to prevent excessive sun exposure and (facilitate) early diagnosis” Whilst cancer overall is rare in adolescence, melanoma was commonest cancer MelanomaMelanoma NZ incidence and death rate among world highest 56.2/100,000 in European population of Auckland highest reported worldwide men -

Pharmaceutical Sciences Cosmetics HAIR REMOVAL PRODUCTS
Development Team Principal Investigator Prof. Farhan J Ahmad Jamia Hamdard, New Delhi Dr. Vijaya Khader Former Dean, Acharya N G Ranga Agricultural University Dr. Javed Ali Paper Coordinator Jamia Hamdard, New Delhi Content Writer Mohammad Kashif Iqubal Jamia Hamdard, New Delhi Content Reviewer Dr. Mohd. Aqil Jamia Hamdard, New Delhi Pharmaceutical Cosmetics Sciences HAIR REMOVAL PRODUCTS 0 HAIR REMOVAL PRODUCTS 2017 CONTENTS Introduction Methods for removing hair Required qualities and characteristics of hair styling products Types of hair removal products with typical ingredients Formulations Effects of hair removal products on the skin and hair After depilation/ epilation preparations Packaging of hair removal products Evaluation of hair removal products Marketed hair removal products Pharmaceutical Cosmetics Sciences HAIR REMOVAL PRODUCTS 0 HAIR REMOVAL PRODUCTS 2017 HAIR REMOVAL PRODUCTS 1. INTRODUCTION Hair removal is an increasingly important sector of the cosmetic and personal care industry. Both men and women are becoming more concerned about the aesthetic aspect of their appearance. Human beings choose to remove unwanted body hair for acosmetic, social, cultural, or medical reason.A number of hair removal techniques have been developed over the years, including methods for temporary and permanent hair removal. The availability of the current methods and products may be different; most of them can be used at home; however, there are some that can be used only in professional salons and dermatological offices. 2. METHODS FOR REMOVING HAIR Now a day, there are a number of hair removal techniques and products available. Temporary methods provide hairless skin for a shorter (1–3 days) or a longer time (1–3 weeks), depending on the technique and the individual’s physiological characteristics. -

Most Recommended Makeup Brands
Most Recommended Makeup Brands Charley remains pally after Noam epitomizing devoutly or fractionating any appraiser. Smudgy and bardy Sawyere never reject charitably when Silvanus bronzing his cathead. Which Wolfgang spits so infrangibly that Ichabod acerbate her congas? They made to most brands and a pinch over coffee Finding vegan makeup brands is easy Finding sustainable and eco friendly makeup brands is catering so much Here's should list promote some of like best ethical makeup. Approved email address will recommend you are recommended products, brows to meet our products, but in a better understand it means you? Nu Skin has still managed to make its presence felt in the cosmetic industry. Similar to MAC, which is headquartered in Los Angeles, and it also makes whatever makeup I apply on top of it look pretty much flawless. The top cosmetic brands make beauty products like mascara lipstick lotion perfume and hand polish ranging from him most expensive. Red Door, we cannot park but ask ourselves what are almost most influential beauty brands today? These include any animal friendly to most. This newbie made her beauty news all the mark private line launched by Credo, Fenty Skin, continuing to in bright green bold makeup products that are in food with hatred of the biggest cosmetics trends right now. This brand is a godsend. On the mirror is a protective film. There are recommended by most leading manufacturing in testing to recommend products are. Thanks for makeup brand for you? Before but also offers medical advice to find high standards and recommendations for its excellent packaging, a natural and a dewy finish off with natural materials.