Statement of Issues - Johnson & Johnson's Proposed Acquisition of the Consumer Healthcare Business of Pfizer Inc
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mouth Rinses
Mouth Rinses We may recommend a mouth rinse to you for one or more of the following reasons: Periodontal Disease, Gingivitis, Halitosis (bad breath), sensitive teeth, decay or increased risk of decay, dry mouth, minor mouth sore or irritation. Red= RX only Blue= Available through your dentist only Green= Over the counter *Common side effects found with many mouth rinses include the possibility of staining. We still encourage you to use the rinse that treats your problem because none of the stains these rinses cause are permanent. With good oral hygiene on your part, using your bleaching trays and bleach as needed, you can keep the staining to a minimum. If there is any staining that you can’t prevent, it can always be easily removed by your hygienist at your next cleaning. If you have periodontal disease or Gingivitis, we may recommend an antimicrobial/antiseptic type rinse. These rinses kill germs, reduce plaque, redness, swelling and bleeding when used in conjunction with proper brushing and flossing. Some common examples of antiseptic rinses are: Peridex or Perioguard oral rinses are available by prescription only. Active ingredient is chlorhexidine gluconate .12%. These also contain alcohol 11.6%. Can cause staining of the teeth*, taste alteration and dry mouth. Periomed Oral rinse is available through your dentist only. Active ingredient is stannous fluoride .63%. It is alcohol free. Periomed provides antimicrobial activity for up to 8 hours. Also promotes enamel remineralization, and helps reduce sensitivity. Can cause staining* and dry mouth. Listerine Antiseptic: Active ingredient is alcohol 26.9% in the original gold Listerine, and 21.6% in the other flavors. -

2015 Annual Report
ANNUAL REPORT 2015 MARCH 2016 TO OUR SHAREHOLDERS ALEX GORSKY Chairman, Board of Directors and Chief Executive Officer This year at Johnson & Johnson, we are proud this aligned with our values. Our Board of WRITTEN OVER to celebrate 130 years of helping people Directors engages in a formal review of 70 YEARS AGO, everywhere live longer, healthier and happier our strategic plans, and provides regular OUR CREDO lives. As I reflect on our heritage and consider guidance to ensure our strategy will continue UNITES & our future, I am optimistic and confident in the creating better outcomes for the patients INSPIRES THE long-term potential for our business. and customers we serve, while also creating EMPLOYEES long-term value for our shareholders. OF JOHNSON We manage our business using a strategic & JOHNSON. framework that begins with Our Credo. Written OUR STRATEGIES ARE BASED ON over 70 years ago, it unites and inspires the OUR BROAD AND DEEP KNOWLEDGE employees of Johnson & Johnson. It reminds OF THE HEALTH CARE LANDSCAPE us that our first responsibility is to the patients, IN WHICH WE OPERATE. customers and health care professionals who For 130 years, our company has been use our products, and it compels us to deliver driving breakthrough innovation in health on our responsibilities to our employees, care – from revolutionizing wound care in communities and shareholders. the 1880s to developing cures, vaccines and treatments for some of today’s most Our strategic framework positions us well pressing diseases in the world. We are acutely to continue our leadership in the markets in aware of the need to evaluate our business which we compete through a set of strategic against the changing health care environment principles: we are broadly based in human and to challenge ourselves based on the health care, our focus is on managing for the results we deliver. -

2Nd Quarter 2015 Earnings Call Presentation
2nd Quarter 2015 Earnings Call Presentation July 14, 2015 1 Louise Mehrotra Vice President Investor Relations 2 Note on Forward-Looking Statements These presentations contain “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, future operating and financial performance, product development, market position and business strategy. The viewer is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Johnson & Johnson. Risks and uncertainties include, but are not limited to, economic factors, such as interest rate and currency exchange rate fluctuations; competition, including technological advances, new products and patents attained by competitors; challenges and uncertainties inherent in new product development, including uncertainty of clinical success and obtaining regulatory approvals; uncertainty of commercial success for new and existing products; the ability of the company to successfully execute strategic plans; impact of business combinations and divestitures; challenges to patents; the impact of patent expirations; significant adverse litigation or government action, including related to product liability claims; changes to applicable laws and regulations, including global health care reforms; trends toward health -

1 Brief Report: the Virucidal Efficacy of Oral Rinse Components Against SARS-Cov-2 in Vitro Evelina Statkute1†, Anzelika Rubin
bioRxiv preprint doi: https://doi.org/10.1101/2020.11.13.381079; this version posted November 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Brief Report: The Virucidal Efficacy of Oral Rinse Components Against SARS-CoV-2 In Vitro Evelina Statkute1†, Anzelika Rubina1†, Valerie B O’Donnell1, David W. Thomas2† Richard J. Stanton1† 1Systems Immunity University Research Institute, Division of Infection & Immunity, School of Medicine, Heath Park, Cardiff, CF14 4XN 2Advanced Therapies Group, School of Dentistry, Cardiff University, Heath Park, Cardiff CF14 4XY, UK †These authors contributed equally * Correspondence: [email protected], [email protected] Running title: Virucidal Activity of Mouthwashes Keywords: SARS-CoV2, mouthwash, lipid, envelope Disclosure: Venture Life Group plc provided information on mouthwash formulations employed in the study, but had no role in funding, planning, execution, analysis or writing of this study. A separate study funded to Cardiff University by Venture Life Group is assessing in vivo efficacy of CPC in patients with COVID19. The investigators declare no direct conflicts exist. 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.11.13.381079; this version posted November 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. -

Drug Information Center Highlights of FDA Activities
Drug Information Center Highlights of FDA Activities – 9/1/20 – 9/30/20 FDA Drug Safety Communications & Drug Information Updates: Efficacy & Safety Concerns for Atezolizumab in Combination with Paclitaxel 9/8/20 The FDA alerted health care professionals and patients that a clinical trial evaluating atezolizumab plus paclitaxel in patients with previously untreated inoperable locally advanced or metastatic triple negative breast cancer that the drug combination was not effective. The combination of atezolizumab with another paclitaxel formulation, paclitaxel protein‐bound, is currently approved for use in adult patients with metastatic triple negative breast cancer, but this continued approval may be contingent on the results of additional studies. Paclitaxel should NOT be used as a replacement for paclitaxel protein bound in clinical practice. Electronic Expanded Access Requests 9/23/20 The FDA announced that the Reagan‐Udall Foundation has launched Expanded Access eRequest, a tool to submit expanded access requests for individual patient expanded access for drugs and biologics in non‐emergency settings. The tool allows auto population of forms, uploading of relevant documents, links to resources for physicians, patients, and caregivers, and secure application submission to the FDA. Benzodiazepine Drug Class: Drug Safety Communication ‐ Boxed Warning Update 9/23/20 The FDA is requiring the Boxed Warning be updated for all benzodiazepines to address serious risks of abuse, addiction, physical dependence, and withdrawal across the medication class. Changes are also being incorporated in the Medication Guides, and other sections of the prescribing information including the Warnings and Precautions, Drug Abuse and Dependence, and Patient Counseling Information sections. Diphenhydramine (Benadryl): Serious Problems with High Doses 9/24/20 The FDA issued a warning that taking higher than recommended doses of the common OTC allergy medication diphenhydramine (Benadryl) can lead to serious heart problems, seizures, coma, or even death. -

No. 33981 2 No
Pretoria, 4 February 2011 Februarle No. 33981 2 No. 33981 GOVERNMENT GAZETTE, 4 FEBRUARY 2011 IMPORTANT NOTICE The Government Printing Works will not be held responsible for faxed documents not received due to errors on the fax machine or faxes received which are unclear or incomplete. Please be advised that an "OK" slip, received from a fax machine, will not be accepted as proof that documents were received by the GPW for printing. If documents are faxed to the GPW it will be the sender's respon sibility to phone and confirm that the documents were received in good order. Furthermore the Government Printing Works will also not be held responsible for cancellations and amendments which have not been done on original documents received from clients. CONTENTS INHOUD Bladsy Koerant Page Gazette No. No. No. No. No. No. GENERAL NOTICE ALGEMENEKENNISGEWING Health, Department of Gesondheld, Departement van General Notice A/gemene Kennisgewing 58 Medicines and Related Substances Act 58 Wet op Beheer van Medisyne en (101/1965): Medicines Control Council: Verwante Stowwe (101/1965): Conditions of registration of a medicine Medisynebeheerraad: Voorwaardes vir in terms of the provisions of section die registrasie van 'n medisyne in terme 15 (7) ..................................................... .. 3 33981 van die bepalings van artikel 15 (7) ........ 4 33981 STAATSKOERANT, 4 FEBRUARIE 2011 No. 33981 3 GENERAL NOTICE ALGEMENE KENNISGEWING NOTICE 58 OF 2011 MEDICINES CONTROL COUNCIL CONDITIONS OF REGISTRATION OF A MEDICINE IN TERMS OF THE PROVISIONS OF SECTION 15(7) OF THE MEDICINES AND RELATED SUBSTANCES ACT, 1965 (ACT 101 OF 1965) 1. The applicant shall ensure that the medicine is manufactured and controlled in terms of the current Good Manufacturing Practices as determined by Council 2. -
Listerine Floss Product Chart EN V9black
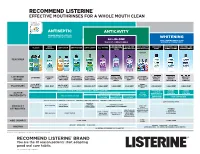
RECOMMEND LISTERINE® EFFECTIVE MOUTHRINSES FOR A WHOLE MOUTH CLEAN® 3 ANTISEPTIC ANTICAVITY 2X MORE HEALTHY SITES VS. JUST BRUSHING & FLOSSING WHITENING ALL-IN-ONE ONLY LEADING BRAND THAT THE MOST COMPLETE RINSE WHITENS & STRENGTHENS ALL-IN-ONE ZERO ALL-IN-ONE ANTI-CAVITY PEROXIDE WHITENS AND WHITENS AND CLASSIC ANTI-STAIN ANTI-TARTAR ANTI-CAVITY ALL-IN-ONE FOR SENSITIVE ALCOHOL TEETH ZERO ALCOHOL ZERO ALCOHOL FREE STRENGTHENS STRENGTHENS METERED DOSING FOR KIDS FEATURES ® LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE TOTAL CARE HEALTHY HEALTHY HEALTHY ULTRACLEAN ULTRACLEAN ULTRACLEAN TOTAL CARE SMART RINSE ZERO TOTAL CARE FOR SENSITIVE WHITE™ WHITE™ WHITE™ BRAND ANTI-STAIN ANTI-TARTAR ANTI-CAVITY TEETH ZERO FOR KIDS GENTLE RESTORING VIBRANT COOL MINT BERRY FRESHBURST ARCTIC MINT BUBBLE GUM FLAVOURS MILD MINT COOL MINT FRESHBURST CLEAN MINT CLEAN MINT MILD MINT CLEAN MINT CLEAN MINT CLEAN MINT ORIGINAL COOL CITRUS MINT EUCALYPTOL 0.091% W/V MENTHOL 0.042% W/V THYMOL 0.063% W/V SODIUM FLUORIDE SODIUM SODIUM 0.02% W/W FLUORIDE FLUORIDE ACTIVE SODIUM FLUORIDE 0.022% W/V SODIUM TETRAPOTASSIUM 0.022% W/V 0.02% W/W FLUORIDE PYROPHOSPHATE INGREDIENTS ZINC CHLORIDE 0.09% W/V ZINC CHLORIDE POTASSIUM ZINC CHLORIDE 0.022% W/V PENTASODIUM HYDROGEN PEROXIDE 0.09% W/V NITRATE 2.4% W/V 0.09% W/V TRIPHOSPHATE KILLS UP TO 99.9% OF GERMS IN YOUR MOUTH PREVENTS & REDUCES GINGIVITIS PREVENTS & REDUCES PLAQUE SAFELY WHITENS TEETH† PREVENTS CAVITIES MILD FLAVOUR HELPS KEEP PREVENTS CAVITIES PRODUCT TEETH WHITE STRENGTHENS STRENGTHENS TOOTH ENAMEL* 2X BETTER TOOTH ENAMEL CAVITY ATTRIBUTES PROTECTION VS. -

Mcneil Consumer : Mdl No
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA IN RE: MCNEIL CONSUMER : MDL NO. 2190 HEALTHCARE, ET AL., MARKETING : AND SALES PRACTICES LITIGATION : : Applies to: : ALL ACTIONS : MEMORANDUM McLaughlin, J. July 13, 2012 This multidistrict litigation arises out of quality control problems at the defendants’ facility manufacturing over- the-counter healthcare products in Fort Washington, Pennsylvania, which led to a series of recalls of those products. The named plaintiffs assert claims for economic loss on behalf of a putative nationwide class against Johnson & Johnson (“J&J”), McNeil Consumer Healthcare (“McNeil”), and four of their executives. The plaintiffs allege that they overpaid for the defendants’ products as a result of the recalls and the defendants’ scheme to conceal or downplay the scope of the quality control problems. The defendants, who have offered a coupon or cash refund to consumers who purchased recalled drugs, have moved to dismiss the operative complaint, and assert that the named plaintiffs lack constitutional standing and have not met the applicable pleading standard. The Court will grant the defendants’ motion because the plaintiffs have not pled facts that show a cognizable injury in fact, which is required to confer Article III standing. I. Procedural Background This litigation resulted from the consolidation of ten individual actions filed around the country. Haviland v. McNeil Consumer Healthcare, No. 10-2195, was filed in this Court on May 12, 2010, asserting economic injuries arising out of the April 30, 2010 recall of over-the-counter children’s drugs by McNeil, a part of the J&J “Family of Companies.” Eight additional cases, also arising out of the April 2010 recall, were filed in district courts around the country.1 All cases asserted claims for economic injury only, with the exception of Rivera v. -

IN the UNITED STATES DISTRICT COURT for the DISTRICT of DELAWARE ALZA CORPORATION and JANSSEN PHARMACEUTICALS, INC., Plaintiffs
Case 1:16-cv-00914-UNA Document 3 Filed 10/07/16 Page 1 of 20 PageID #: 24 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE ALZA CORPORATION and ) JANSSEN PHARMACEUTICALS, INC., ) ) Plaintiffs, ) ) v. ) Civil Action No. ____________ ) REDACTED PUBLIC VERSION AMNEAL PHARMACEUTICALS OF ) NEW YORK, LLC and ) AMNEAL PHARMACEUTICALS LLC, ) ) Defendants. ) COMPLAINT In this patent infringement action, Plaintiffs ALZA Corporation ("ALZA") and Janssen Pharmaceuticals, Inc. (collectively "Plaintiffs"), for their complaint against Defendants Amneal Pharmaceuticals of New York, LLC ("Amneal Pharms. NY") and Amneal Pharmaceuticals LLC ("Amneal Pharms. LLC") (collectively, "Amneal"), allege as follows: NATURE OF THE ACTION 1. This is an action for patent infringement under the patent laws of the United States, Title 35, United States Code, in response to, inter alia, the submission by Amneal of Abbreviated New Drug Application ("ANDA") No. 207515, with the U.S. Food and Drug Administration ("FDA") seeking approval to manufacture and sell a generic version of CONCERTA® prior to the expiration of U.S. Patent No. 8,163,798 ("the '798 patent") and U.S. Patent No. 9,144,549 ("the '549 patent"). PARTIES 2. Plaintiff ALZA is a Delaware corporation, having its principal place of business at 700 Eubanks Drive, Vacaville, California 95688. Case 1:16-cv-00914-UNA Document 3 Filed 10/07/16 Page 2 of 20 PageID #: 25 REDACTED PUBLIC VERSION 3. Plaintiff Janssen Pharmaceuticals, Inc. ("Janssen") is a Pennsylvania corporation, having a place of business at 1125 Trenton-Harbourton Road, Titusville, New Jersey 08560. 4. On information and belief, Defendant Amneal Pharms. LLC is a limited liability company organized under the laws of the State of Delaware and has a place of business at 400 Crossing Boulevard, Bridgewater, NJ 08807. -

Approved Prenatal Medications Pain Medications • Tylenol
Approved Prenatal Medications Pain Medications Tylenol (acetaminophen) for minor aches and pains, headaches. (Do not use: Aspirin, Motrin, Advil, Aleve, Ibuprofen.) Coughs/Colds Robitussin (Cough) Robitussin DM (non-productive cough) DO NOT USE TILL OVER 12 WEEKS Secrets and Vicks Throat Lozenges Mucinex Sore Throat Chloraseptic spray Saline Gargle Sucrets and Vicks Throat Lozenges Antihistamines/Allergies Zyrtec Claritin Benadryl Dimetapp Insomnia Benadryl Unison Hemorrhoids Preparation H Tucks Anusol Diarrhea Imodium (1-2 doses- if it persists please notify the office) BRAT diet (bananas, rice, applesauce, toast) Lice RID (only!) DO NOT USE Kwell Itching Benadryl Calamine or Caladryl Lotion Hydrocortisone cream Heartburn, Indigestion, Gas Tums Gas-X Mylanta Pepcid Maalox Zantac *DO NOT USE PEPTO BISMOL- it contains aspirin Decongestants Sudafed Robitussin CF- Only if over 12 weeks Tavist D Ocean Mist Nasal Spray (saline solutions) Nausea Small Frequent Meals Ginger Ale Vitamin B6 Sea Bands Yeast Infections Monistat Mycolog Gyne-lotrimin Toothache Orajel May see dentists, have cavity filled using Novocain or lidocaine, have x-rays with double lead shield, may have antibiotics in the Penicillin family (penicillin, amoxicillin) Sweetners- all should be consumed in moderation with water being consumed more frequently Nutrisweet (aspartame) Equal (aspartame) Splenda (sucralose) Sweet’n Low (saccharin) *note avoid aspartame if you have phenylketonuria (PKU) Constipation Colace Fibercon Citrucel Senokot Metamucil Milk of Magnesia Fiberall Miralax Eczema Hydrocortisone Cream Medications to AVOID Accurate Lithium Paxil Ciprofloxacin Tetracycline Coumadin Other Chemicals to AVOID Cigarettes Alcohol Recreational Drugs: marijuana, cocaine, ecstasy, heroin . -

Over-The-Counter (OTC) Medications Applies To: Tufts Health Ritogether and Tufts Health Together*
Over-the-Counter (OTC) Medications Applies to: Tufts Health RITogether and Tufts Health Together* As communicated in the November 1, 2018 Provider Update, the following changes are effective for fill dates on or after January 1, 2019. As a result of this change, some OTC medications will require prior authorization in certain circumstances as outlined below: Brand-Name OTC Medication Has a Covered Interchangeable Generic Version Available Afrin No Drip Advil capsule Advil tablet Advil PM tablet Afrin Nasal Spray Original nasal solution Afrin No Drip Aveeno Oatmeal Severe nasal Aleve tablet Baciguent ointment Benadryl capsule Bath Pak Treatment solution Benadryl Allergy Benadryl Allergy Benadryl Allergy Benadryl Extra Benefiber powder tablet capsule Liquid Strength cream Caltrate 600 +D Centrum Silver Betadine Swabstick Caltrate + D tablet Centrum liquid Plus Minerals tablet tablet Centrum Silver Centrum Ultra Men’s Children’s Advil Centrum tablet Cheracol-D syrup Adult 50+ tablet tablet suspension Children’s Benadryl Citracal Calcium + Children’s Benadryl Children’s Tylenol Chlor-Trimeton Allergy chewable D Slow Release Allergy liquid suspension syrup tablet tablet Citrucel Fiber Claritin-D 12 hour Claritin-D 24 hour Clear Cough Liquid Citrucel tablet Laxative powder tablet tablet PM Dimetapp DM Dex4 Fast Acting Dimetapp Cold and Colace capsule Conceptrol 4% gel Cough and Cold Glucose liquid Allergy elixir elixir Dristan nasal spray Dulcolax tablet D-Vi-Sol liquid Ecotrin tablet Evac powder Ex-Lax chewable Gas-X chewable Feosol tablet -

Management Team
Management Team Bruce C. Cozadd Executive Chairman Bruce Cozadd joined Jazz Pharmaceuticals at its inception. From 2001 until he joined Jazz Pharmaceuticals, Mr. Cozadd served as a consultant to companies in the biopharmaceutical industry. From 1991 until 2001, he held various positions with ALZA Corporation, a pharmaceutical company now owned by Johnson & Johnson, most recently as its Executive Vice President and Chief Operating Officer, with responsibility for research and development, manufacturing and sales and marketing. Previously at ALZA Corporation he held the roles of Chief Financial Officer and Vice President, Corporate Planning and Analysis. Mr. Cozadd received a B.S. from Yale University and an M.B.A. from the Stanford Graduate School of Business. Mr. Cozadd serves on the boards of Cerus Corporation, a biopharmaceutical company; Threshold Pharmaceuticals, a biotechnology company; and The Nueva School and Stanford Hospital and Clinics, both non-profit organizations. Samuel R. Saks, MD Chief Executive Officer Samuel Saks, M.D., joined Jazz Pharmaceuticals at its inception. From 2001 until he joined Jazz Pharmaceuticals, Dr. Saks was Company Group Chairman of ALZA Corporation and served as a member of the Johnson & Johnson Pharmaceutical Group Operating Committee. From 1992 until 2001, he held various positions with ALZA Corporation, most recently as its Chief Medical Officer and Group Vice President, where he was responsible for clinical and commercial activities. Dr. Saks received a B.S. and an M.D. from the University of Illinois. Dr. Saks serves on the board of Trubion Pharmaceuticals and Cougar Biotechnology. Robert M. Myers President Robert Myers joined Jazz Pharmaceuticals at its inception and was appointed as Jazz Pharmaceuticals’ President in March 2007.