Standard Plate Count, Coliform, and Simplified Count Methods
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Microbiology Laboratory Exercises Third Edition 2020
MICROBIOLOGY Laboratory Exercises Third Edition Keddis & Rauschenbach 2020 Photo Credits (in order of contribution): Diane Davis, Ines Rauschenbach & Ramaydalis Keddis Acknowledgements: Many thanks to those in the Department of Biochemistry and Microbiology, Rutgers University, who have through the years inspired our enthusiasm for the science and teaching of microbiology, with special thanks to Diane Davis, Douglas Eveleigh and Max Häggblom. Safety: The experiments included in this manual have been deemed safe by the authors when all necessary safety precautions are met. The authors recommend maintaining biosafety level 2 in the laboratory setting and using risk level 1 organisms for all exercises. License: This work is licensed under a Creative Commons Attribution- NonCommercial-NoDerivatives 4.0 International License Microbiology Laboratory Exercises Third Edition 2020 Ramaydalis Keddis, Ph.D. Ines Rauschenbach, Ph.D. Department of Biochemistry and Microbiology Rutgers, The State University of New Jersey CONTENTS PAGE Introduction Schedule ii Best Laboratory Practices Iii Working in a Microbiology Laboratory iv Exercises Preparation of a Culture Medium 1 Culturing and Handling Microorganisms 3 Isolation of a Pure Culture 5 Counting Bacterial Populations 8 Controlling Microorganisms 10 Disinfectants 10 Antimicrobial Agents: Susceptibility Testing 12 Hand Washing 14 The Lethal Effects of Ultraviolet Light 15 Selection of Fungi from Air 17 Microscopy 21 Morphology and Staining of Bacteria 26 Microbial Metabolism 30 Enzyme Assay 32 Metabolic -
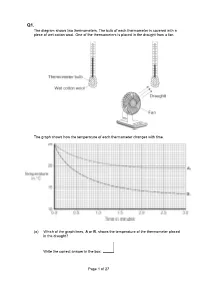
Page 1 of 27 the Diagram Shows Two Thermometers. the Bulb of Each
Q1. The diagram shows two thermometers. The bulb of each thermometer is covered with a piece of wet cotton wool. One of the thermometers is placed in the draught from a fan. The graph shows how the temperature of each thermometer changes with time. (a) Which of the graph lines, A or B, shows the temperature of the thermometer placed in the draught? Write the correct answer in the box. Page 1 of 27 Explain, in terms of evaporation, the reason for your answer. ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ (3) (b) A wet towel spread out and hung outside on a day without wind dries faster than an identical wet towel left rolled up in a plastic bag. Explain why. ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ (2) (Total 5 marks) Q2. The picture shows a person taking a hot shower. (a) When a person uses the shower the mirror gets misty. Why? ___________________________________________________________________ ___________________________________________________________________ -

Pouring Plates from Prepared Bottled Media
Pouring Plates from Prepared Bottled Media Primary Hazard Warning Never purchase living specimens without having a disposition strategy in place. When pouring bottles, agar is HOT! Burning can occur. Always handle hot agar bottles with heat-protective gloves. For added protection wear latex or nitrile gloves when working with bacteria, and always wash hands before and after with hot water and soap. Availability Agar is available for purchase year round. Information • Storage: Bottled agar can be stored at room temperature for about six months unless otherwise specified. Never put agar in the freezer. It will cause the agar to breakdown and become unusable. To prevent contamination keep all bottles and Petri dishes sealed until ready to use. • Pouring Plates • Materials Needed: • Draft-free enclosure or Laminar flow hood • 70% isopropyl alcohol • Petri dishes • Microwave or hot water bath or autoclave 1. Melt the agar using one of the following methods: a) Autoclave: Loosen the cap on the agar bottle and autoclave the bottle at 15 psi for five minutes. While wearing heat-protective gloves, carefully remove the hot bottle and let it cool to between 75–55°C before pouring. This takes approximately 15 minutes. b)Water Bath: Loosen the cap on the agar bottle and place it into a water bath. Water temperature should remain at around 100°C. Leave it in the water bath until the agar is completely melted. While wearing heat- protective gloves, carefully remove the hot bottle and let it cool to between 75–55°C before pouring. c) Microwave: Loosen the cap on the agar bottle before microwaving. -

METHODS & STANDARD OPERATING PROCEDURES (Sops)
METHODS & STANDARD OPERATING PROCEDURES (SOPs) OF EMISSION TESTING IN HAZARDOUS WASTE INCINERATOR FOREWARD COVR PAGE TEAM CHAPTER TITLE PAGE NO Chapter - 1 Stack Monitoring – Material And Methodology For Isokinetic Sampling 1-28 Chapter - 2 Standard Operating Procedure (SOP) For Particulate Matter Determination 29-38 Chapter - 3 Determination Of Hydrogen Halides (Hx) And Halogens From Source Emission 39-54 Chapter - 4 Standard Operating Procedure For The Sampling Of Hydrogen Halides And Halogens From Source Emission 55-61 Determination Of Metals And Non Metals Emissions From Chapter - 5 Stationarysources 62-79 Standard Operating Procedure (Sop) For Sampling Of Metals And Non Chapter - 6 Metals & 80-97 Standard Operating Procedure (Sop) Of Sample Preparation For Analysis Of Metals And Non Metals Chapter - 7 Determination Of Polychlorinated Dibenzo-P-Dioxins And 98-127 Polychlorinated Dibenzofurans Standard Operating Procedure For Sampling Of Polychlorinated Chapter - 8 Dibenzo-P-Dioxins And Polychlorinated Dibenzofurans & 128-156 Standard Operating Procedure For Analysis Of Polychlorinated Dibenzo- P-Dioxins And Polychlorinated Dibenzofurans Project Team 1. Dr. B. Sengupta, Member Secretary - Overall supervision 2. Dr. S.D. Makhijani, Director - Report Editing 3. Sh. N.K. Verma, Ex Additional Director - Project co-ordination 4. Sh. P.M. Ansari, Additional Director - Report Finalisation 5. Dr. D.D. Basu, Senior Scientist - Guidance and supervision 6. Sh. Paritosh Kumar, Sr. Env. Engineer - Report processing 7. Sh. Dinabandhu Gouda, Env. Engineer - Data compilation, analysis and preparation of final report 8. Sh. G. Thirumurthy, AEE - - do - 9. Sh. Abhijit Pathak, SSA - - do - 10. Ms. Trapti Dubey, Jr. Professional (Scientist) - - do - 11. Er. Purushotham, Jr. Professional (Engineer) - - do - 12. -

Microlab® STAR™
Microlab ® STAR™ Microlab ® STAR ™ AUTOMATED WORKFLOW SOLUTIONS CENTERED AROUND YOUR ASSAY The STAR combines Hamilton's patented pipetting technology including precise lock-and-key tip attachment, unrivaled liquid level detection, and comprehensive volume ranges to create flexible liquid handling workstations. Available in three base platform sizes, the STAR portfolio incorporates countless options to automate your workflows. Hamilton Robotics has also partnered with top leaders in the biotechnology industry to provide Standard Solutions based on commonly automated applications. Offering ready-to-start protocols for a variety of applications such as NGS, ELISA, and forensic assays, our Standard Solutions provide a faster way to automate your processes. 2 1 PATENTED TECHNOLOGY The STAR utilizes Hamilton’s proprietary Compressed O-Ring Expansion (CO-RE®) technology. CO-RE minimizes the production of aerosols and allows disposable tips or washable, steel needles to be used on channels in the same run. 2 MULTI-FUNCTIONAL ARM Our technology offers high pipetting accuracy and precision, from sub-microliter to large volumes, using Independent Channels and/or the Multi-Probe Head (MPH). Labware transportation is possible with the iSWAP® or CO-RE Grippers. The STAR can incorporate a camera, tube transportation, and other channel tools on a single arm. Comprehensive pipetting range: ■■■0.5 μL to 1 mL using the 1 mL Independent Channel ■■■50 μL to 5 mL using the 5 mL Independent Channel ■■■1 μL to 1 mL using the CO-RE 96 MPH ■■■0.1 μL to 50 μL using the CO-RE 384 MPH 3 FLEXIBLE SETUP The high-capacity deck is customized specific to your workflow, accommodating a wide range of labware and automated devices that can easily be exchanged to support multiple assays on one platform. -

Eight New Filter Bags from Whirl-Pak® Three New Hydrated Sponge
NEW PRODUCTS Eight new filter bags from whirl-pak® Specially designed for use with homogenizer blenders. Extra-heavy PET film and special features for easier and more efficient testing. Additional sizes and styles on page 11. Vertical Filter Bags Bags use a special laminated PET film for durability and a vertical nonwoven filter to separate solids for easy pipetting of the liquid sample. Available with or without a closure, each bag contains a 250 micron polyester filter adhered vertically along the side of the 3 mil (0.76 mm) bag. Easy-pour side seal allows you to simply pour liquid out of the bag without also emptying solids. Hydrated PolySponge™ Bags B01586 — 55 oz. With Closure three new Hydrated sponge products from whirl-pak® Make environmental surface sampling faster and easier with these pre-moistened bags. Durable high-density polyurethane sponge is ready to use and resists tearing and fraying. Each is hydrated with HiCap™ Neutralizing Broth. Additional information on page 12. Hydrated PolyProbe™ HiCap™ is a registered trademark of World Bioproducts, LLC. ctured under a ufa qua n li Whirl-Pak® Bags are manufactured under a quality management system certified to ISO9001, except B01299, B01350, a t y M m o , , , , , , , and which require an additional processing step an t B01392 B01422 B01423 B01475 B01478 B01590 B01591 B01592 age fied ISO9001ment system certi done outside of regular production. • Standard Methods Says — Standard Methods (18th edition, 1992, section 9060A, sample containers) states “For some applications, samples may be collected in pre-sterilized plastic bags.” Whirl-Pak® bags meet this description. -

Manual on Laboratory Testing of Fishery Products
ISSN: 1995-4875 CRFM Special Publication. No.14 Manual on Laboratory Testing of Fishery Products The SPS Project is funded by the European Union under the 10th Economic Development Fund and is being implemented by the Inter-American Institute for Cooperation on Agriculture (IICA) with the following regional Partners: the CARICOM Secretariat, the Caribbean Regional Fisheries Mechanism (CRFM), El Comité Nacional para la Aplicació n de Medidas Sanitarias y Fitosanitarias de la Repú blica Dominicana (CNMSF) and CARIFORUM. Manual on Laboratory Testing of Fisheries Products Copyright © 2016 by Caribbean Regional Fisheries Mechanism (CRFM) All rights reserved. Reproduction, dissemination and use of material in this publication for educational or non- commercial purposes are authorized without prior written permission of the CRFM, provided the source is fully acknowledged. No part of this publication may be reproduced, disseminated or used for any commercial purposes or resold without the prior written permission of the CRFM. Prepared by: Christine Froese, Megapesca Lda., December 2016, under contract to the Inter- American Institute for Cooperation on Agriculture (IICA), through the 10th EDF funded Sanitary and Phytosanitary Project Correct Citation: Froese, C, 2016. Manual on Laboratory Testing of Fishery Products. CRFM Special Publication. No.14. 89pp. ISSN: 1995-4875 ISBN: 978-976-8257-38-3 Cover Photo: Fishery Product Testing Laboratory, Saint Vincent Contents 1 INTRODUCTION ............................................................................................................................... -

Evobot: an Open-Source, Modular, Liquid Handling Robot for Scientific Experiments
applied sciences Article EvoBot: An Open-Source, Modular, Liquid Handling Robot for Scientific Experiments Andres Faiña 1,* , Brian Nejati 2 and Kasper Stoy 1 1 Robots, Evolution and Art Lab (REAL), Department of Computer Science, IT University of Copenhagen, 2300 Copenhagen, Denmark; [email protected] 2 Department of Mechanical and Industrial Engineering, University of Toronto, Toronto, ON M5S 1A1, Canada; His work was carried out while he was employed at ITU; [email protected] * Correspondence: [email protected]; Tel.: +45-7218-5258 Received: 13 October 2019; Accepted: 17 January 2020; Published: 23 January 2020 Featured Application: Our modular liquid handling robot can be applied to automate scientific experiments in research labs, where tasks change often, and to carry out new kinds of experiments that cannot be done manually. Abstract: Commercial liquid handling robots are rarely appropriate when tasks change often, which is the case in the early stages of biochemical research. In order to address it, we have developed EvoBot, a liquid handling robot, which is open-source and employs a modular design. The combination of an open-source and a modular design is particularly powerful because functionality is divided into modules with simple, well-defined interfaces, hence customisation of modules is possible without detailed knowledge of the entire system. Furthermore, the modular design allows end-users to only produce and assemble the modules that are relevant for their specific application. Hence, time and money are not wasted on functionality that is not needed. Finally, modules can easily be reused. In this paper, we describe the EvoBot modular design and through scientific experiments such as basic liquid handling, nurturing of microbial fuel cells, and droplet chemotaxis experiments document how functionality is increased one module at a time with a significant amount of reuse. -

Process Skills Review Warm-Up C
Process Skills Review Warm-Up C. • Define function • Match the following 1. Puts out fire 2. Curved line of liquid in a graduated cylinder 3. Used to observe insects A. B. Which of the following describes the correct way to handle chemicals in a laboratory? A. It is safe to combine unknown chemicals as long as only small amounts are used. B. Return all chemicals to their original containers. C. Always pour extra amounts of the chemicals called for the experiment. D. To test for odors, always use a wafting motion. Which of the following describes the correct way to handle chemicals in a laboratory? A. It is safe to combine unknown chemicals as long as only small amounts are used. B. Return all chemicals to their original containers. C. Always pour extra amounts of the chemicals called for the experiment. D. To test for odors, always use a wafting motion. What task is being performed in the picture? A. measuring mass with a graduated cylinder B. measuring length with a triple beam balance C. measuring mass with a triple beam balance D. measuring length with a metric ruler What task is being performed in the picture? A. measuring mass with a graduated cylinder B. measuring length with a triple beam balance C. measuring mass with a triple beam balance D. measuring length with a metric ruler John and Lisa are conducting an experiment in their 7th grade science class in which they are handling potentially dangerous chemicals. As John is pouring a chemical from a beaker to a graduated cylinder, he splashes some of the chemical into his eyes. -

Building a Morbidostat: an Automated Continuous-Culture Device For
PROTOCOL Building a morbidostat: an automated continuous- culture device for studying bacterial drug resistance under dynamically sustained drug inhibition Erdal Toprak1,2, Adrian Veres3, Sadik Yildiz2, Juan M Pedraza4, Remy Chait1, Johan Paulsson1,5 & Roy Kishony1,5 1Department of Systems Biology, Harvard Medical School, Boston, Massachusetts, USA. 2Faculty of Engineering and Natural Sciences, Sabanci University, Istanbul, Turkey. 3Health Sciences and Technology Program, Harvard Medical School, Boston, Massachusetts, USA. 4Department of Physics, Universidad de los Andes, Bogotá, Colombia. 5School of Engineering and Applied Sciences, Harvard University, Cambridge, Massachusetts, USA. Correspondence should be addressed to E.T. ([email protected]) or R.K. ([email protected]). Published online 21 February 2013; doi:10.1038/nprot.2013.021 We present a protocol for building and operating an automated fluidic system for continuous culture that we call the ‘morbidostat’. The morbidostat is used to follow the evolution of microbial drug resistance in real time. Instead of exposing bacteria to predetermined drug environments, the morbidostat constantly measures the growth rates of evolving microbial populations and dynamically adjusts drug concentrations inside culture vials in order to maintain a constant drug-induced inhibition. The growth rate measurements are done using an optical detection system that is based on measuring the intensity of back-scattered light from bacterial cells suspended in the liquid culture. The morbidostat can additionally be used as a chemostat or a turbidostat. The whole system can be built from readily available components within 2–3 weeks by biologists with some electronics experience or engineers familiar with basic microbiology. INTRODUCTION Antibiotic resistance is an important public health problem, ren- at a constant rate lower than the maximal growth rate of the popu- dering currently available drugs useless and threatening millions lation, the dilution rate of the morbidostat rdilution ≅ ∆V/(V·∆t) is of lives1–4. -

Microbiology. Applied to Protecting the Line
3M Food Safety Division Microbiology. Applied to protecting the line www.3M.com/foodsafety/us Increase Productivity & Profitability to Your Business by 3M Food Safety Product Cost 45% Product Cost Saving Increase Productivity • 45% Reducing labor cost, saving utilities • Ability to increase productivity in food processing. and equipments of sample preparation 3M rapid solution help to detect contamination, • The shelf-stable, thin design takes up 85% less space faster time to results. Making faster decision. than agar dishes, freeing incubator and storage space and significantly reducing biohazardous waste • Control the production process better than ever. Higher product quality and Reduce overall costs, such as reducing waste, reducing Break down. OFF ON Fas te ISO r HACCP GMP Brand Protection Inventory Management • Establish brand credibility. Reliable • Release product to the hands of consumers faster. testing results with the global standard Company faster gaining profit • Reduce risk of product from the Recall • Reduce cost of storage inventory concerns. and Reject. Making trust from business partners. 2 Increase Laboratory Productivity by 3M Food Safety Product Product 3M Food Safety Cost 45% Cost Saving Accuracy • 45% Reduce the cost of labor, • Provide accurate results to Make decisions saving utilities and equipments correctly. Increase effectiveness of laboratory for sample preparation. • Build the credibility of laboratory with your • Reduce the cost of acquisition lab partners and auditors with the international equibments or the expansion of the method standards laboratory. Space less than 85% of Agar. Time 50% Software Time • Faster time to result.The product is designed to reduce steps and timing compared to the traditional method. -

STERILE TECHNIQUE PRACTICE Module 3 Supplement
STUDENT STERILE TECHNIQUE PRACTICE Module 3 Supplement ONLY FOR PRACTICE you should Re-use the loop and pipette and re-use their "sterile" wrappings Do not dispose of a culture dish even if you accidentally contaminate it. If you were really doing a lab with bacteria you would • Never reuse any loop or pipette. • Always dispose of used loops and pipettes in the "biological trash beaker." • Throw away any culture dish or other item that is accidentally contaminated, and ask for a replacement. • Wash hands immediately if they contact any liquids containing bacteria. Each member of the team should practice each of the following: 1. Open, hold, and close a sterile 15 ml culture tube. 2. Open and close a sterile 1.5 ml test tube. 3. Open a culture dish, observe it without breathing on it, and close it. After each team member has practiced 1-3, use (practice) sterile technique as you 4. Transfer 250 l of liquid from the 1.5 ml test tube to the culture tube. 5. Pretend to transfer a colony from the surface of the culture dish to the liquid in the culture tube. 6. Use the pipette to suspend the imaginary bacteria in the culture tube liquid. After each team member has practiced 4-6, use (practice) sterile technique as you 7. Transfer 100 l of culture tube contents to culture dish and spread it. POSSIBLE SEQUENCE OF STEPS FOR STERILE TECHNIQUE DEMONSTRATION/PRACTICE Delete parts as time requires 1. a. Teacher demonstrates opening/holding/closing of sterile 1.5 ml test tube, sterile 15 ml culture tube, sterile pipettes, measuring with pipettes.