Microbiology Laboratory Exercises Third Edition 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Exploring Small-Scale Chemostats to Scale up Microbial Processes: 3-Hydroxypropionic Acid Production in S
Lawrence Berkeley National Laboratory Recent Work Title Exploring small-scale chemostats to scale up microbial processes: 3-hydroxypropionic acid production in S. cerevisiae. Permalink https://escholarship.org/uc/item/43h8866j Journal Microbial cell factories, 18(1) ISSN 1475-2859 Authors Lis, Alicia V Schneider, Konstantin Weber, Jost et al. Publication Date 2019-03-11 DOI 10.1186/s12934-019-1101-5 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Lis et al. Microb Cell Fact (2019) 18:50 https://doi.org/10.1186/s12934-019-1101-5 Microbial Cell Factories RESEARCH Open Access Exploring small-scale chemostats to scale up microbial processes: 3-hydroxypropionic acid production in S. cerevisiae Alicia V. Lis1, Konstantin Schneider1,2, Jost Weber1,3, Jay D. Keasling1,4,5,6,7, Michael Krogh Jensen1 and Tobias Klein1,2* Abstract Background: The physiological characterization of microorganisms provides valuable information for bioprocess development. Chemostat cultivations are a powerful tool for this purpose, as they allow defned changes to one single parameter at a time, which is most commonly the growth rate. The subsequent establishment of a steady state then permits constant variables enabling the acquisition of reproducible data sets for comparing microbial perfor- mance under diferent conditions. We performed physiological characterizations of a 3-hydroxypropionic acid (3-HP) producing Saccharomyces cerevisiae strain in a miniaturized and parallelized chemostat cultivation system. The physi- ological conditions under investigation were various growth rates controlled by diferent nutrient limitations (C, N, P). Based on the cultivation parameters obtained subsequent fed-batch cultivations were designed. Results: We report technical advancements of a small-scale chemostat cultivation system and its applicability for reliable strain screening under diferent physiological conditions, i.e. -

Microscope Compatible Cell-Culture Incubator
Microscope Compatible Cell-Culture Incubator BME 400 December 14th, 2016 Client: Dr. John Puccinelli, UW-Madison Dept. of Biomedical Engineering Advisor: Dr. Mitchell Tyler, UW-Madison Dept. of Biomedical Engineering Team Members: Trevor Zarecki - Team Leader Jenny Westlund - Communicator Peter Hartig - BPAG Jack McGinnity - BWIG Steve Gock - BSAC Abstract Live cell imaging experiments are difficult to perform over long periods of time on normal lab microscopes without incubation capabilities. Current commercial microscope-stage incubators are expensive, not compatible with multiple microscopes and ineffective at evenly controlling the environment. The client desires an inexpensive incubation chamber compatible with cell microscopy that is capable of maintaining desired temperature, CO2, and humidity evenly throughout the chamber. The device should not alter image quality, and should be accessible for changing media or cell culture dishes. An initial prototype has been developed that involves a small, cohesive system to regulate temperature, CO2, and humidity. Testing of the current prototype has demonstrated regulation of these three systems, through an automated feedback system capable of maintaining the system near a physiological set point. Further development of the design will ensure that it performs efficiently according to all specifications, and ultimately help bridge the gap in the market between high-cost, functional incubation systems and cheaper, less effective designs. 1 Table of Contents Introduction 3 Motivation 3 Existing -

Detection of Acid-Producing Bacteria Nachweis Von Säureproduzierenden Bakterien Détection De Bactéries Produisant Des Acides
(19) TZZ ¥ _T (11) EP 2 443 249 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C12Q 1/04 (2006.01) G01N 33/84 (2006.01) 19.11.2014 Bulletin 2014/47 (86) International application number: (21) Application number: 10790013.6 PCT/US2010/038569 (22) Date of filing: 15.06.2010 (87) International publication number: WO 2010/147918 (23.12.2010 Gazette 2010/51) (54) DETECTION OF ACID-PRODUCING BACTERIA NACHWEIS VON SÄUREPRODUZIERENDEN BAKTERIEN DÉTECTION DE BACTÉRIES PRODUISANT DES ACIDES (84) Designated Contracting States: (74) Representative: Isarpatent AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Patent- und Rechtsanwälte GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Friedrichstrasse 31 PL PT RO SE SI SK SM TR 80801 München (DE) (30) Priority: 15.06.2009 US 187107 P (56) References cited: 15.03.2010 US 314140 P US-A- 4 528 269 US-A- 5 098 832 US-A- 5 164 301 US-A- 5 601 998 (43) Date of publication of application: US-A- 5 601 998 US-A- 5 786 167 25.04.2012 Bulletin 2012/17 US-B2- 6 756 225 US-B2- 7 150 977 (73) Proprietor: 3M Innovative Properties Company • DARUKARADHYA J ET AL: "Selective Saint Paul, MN 55133-3427 (US) enumeration of Lactobacillus acidophilus, Bifidobacterium spp., starter lactic acid bacteria (72) Inventors: and non-starter lactic acid bacteria from Cheddar • YOUNG, Robert, F. cheese", INTERNATIONAL DAIRY JOURNAL, Saint Paul, Minnesota 55133-3427 (US) ELSEVIER APPLIED SCIENCE, BARKING, GB, • MACH, Patrick, A. -

Effective Contamination Control with CO2 Incubators
WHITE PAPER No. 30 Effective Contamination Control with CO2 Incubators Ines Kristina Hartmann¹, James Jarvis² ¹Eppendorf AG, ²Eppendorf, Inc. Executive Summary CO2 incubators provide an optimal cell growth environ- ment by maintaining a humidified atmosphere with temperature and carbon dioxide control. These conditions not only promote cell growth, but also the growth of contaminants, like bacteria, yeast, molds and other fungi. The contamination-reducing features of an incubator’s functional design and the effectiveness of its self-decon- tamination system must be considered in choosing an instrument. In this paper, we compare various strategies for preventing contamination in CO2 incubators, from the functional design of the device to self-disinfection programs. We also give some useful tips to prevent contamination when using CO2 incubators. Introduction Sources of contamination How do contaminants get into a CO2 incubator? Contamination is a major cause of frustration when culturing The CO2 incubator may become an indirect source of con- cells. There are many sources of contamination, either direct tamination. Unlike a biological safety cabinet, an incubator or indirect. Direct sources are contaminated reagents, media cannot prevent the influx of airborne contaminants, as the or seed culture. Media and reagents from reputable suppliers door must be opened during routine use. The incubator are rarely delivered contaminated nowadays. New cell lines chamber can also be contaminated by carelessness in aseptic can introduce contaminants into the lab, especially when techniques, and unnoticed splashes from cell culture vessels. they are given from lab to lab. They should be quarantined Some CO2 incubators use a HEPA filter to remove microor- before culturing with the rest of the cells. -

Chemostat Culture for Yeast Experimental Evolution
Downloaded from http://cshprotocols.cshlp.org/ at Cold Spring Harbor Laboratory Library on August 9, 2017 - Published by Cold Spring Harbor Laboratory Press Protocol Chemostat Culture for Yeast Experimental Evolution Celia Payen and Maitreya J. Dunham1 Department of Genome Sciences, University of Washington, Seattle, Washington 98195 Experimental evolution is one approach used to address a broad range of questions related to evolution and adaptation to strong selection pressures. Experimental evolution of diverse microbial and viral systems has routinely been used to study new traits and behaviors and also to dissect mechanisms of rapid evolution. This protocol describes the practical aspects of experimental evolution with yeast grown in chemostats, including the setup of the experiment and sampling methods as well as best laboratory and record-keeping practices. MATERIALS It is essential that you consult the appropriate Material Safety Data Sheets and your institution’s Environmental Health and Safety Office for proper handling of equipment and hazardous material used in this protocol. Reagents Defined minimal medium appropriate for the experiment For examples, see Protocol: Assembly of a Mini-Chemostat Array (Miller et al. 2015). Ethanol (95%) Glycerol (20% and 50%; sterile) Yeast strain of interest Equipment Agar plates (appropriate for chosen strain) Chemostat array Assemble the apparatus as described in Miller et al. (2013) and Protocol: Assembly of a Mini-Chemostat Array (Miller et al. 2015). Cryo deep-freeze labels Cryogenic vials Culture tubes Cytometer (BD Accuri C6) Glass beads, 4 mm (sterile; for plating yeast cells) Glass cylinder Kimwipes 1Correspondence: [email protected] © 2017 Cold Spring Harbor Laboratory Press Cite this protocol as Cold Spring Harb Protoc; doi:10.1101/pdb.prot089011 559 Downloaded from http://cshprotocols.cshlp.org/ at Cold Spring Harbor Laboratory Library on August 9, 2017 - Published by Cold Spring Harbor Laboratory Press C. -
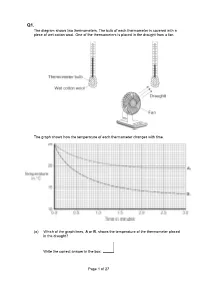
Page 1 of 27 the Diagram Shows Two Thermometers. the Bulb of Each
Q1. The diagram shows two thermometers. The bulb of each thermometer is covered with a piece of wet cotton wool. One of the thermometers is placed in the draught from a fan. The graph shows how the temperature of each thermometer changes with time. (a) Which of the graph lines, A or B, shows the temperature of the thermometer placed in the draught? Write the correct answer in the box. Page 1 of 27 Explain, in terms of evaporation, the reason for your answer. ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ (3) (b) A wet towel spread out and hung outside on a day without wind dries faster than an identical wet towel left rolled up in a plastic bag. Explain why. ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ (2) (Total 5 marks) Q2. The picture shows a person taking a hot shower. (a) When a person uses the shower the mirror gets misty. Why? ___________________________________________________________________ ___________________________________________________________________ -

Maintenance of Bacterial Strains
Maintenance of Bacterial Strains Short-term growth and maintenance The simplest method for obtaining strains is to order them from a microbiological supply company. Presque Isle, Ward, and Carolina Biological (see “Resources” page of the Microbes Web site: www.umsl.edu/~microbes) are economical sources of strains that are commonly used in the classroom. Another possible source is a local college or university that teaches a microbiology lab course. If they have strains already prepared for their class, they will probably be willing to provide you with a culture. This is probably not a reasonable ongoing source of strains, but can be useful in an emergency. For short-term maintenance and use, it is best to streak bacteria on some type of rich agar petri plate, such as nutrient agar. Label and date the plate on the bottom of the plate. Streak from the source culture using good sterile technique. The quadrant streak method (see the article on quadrant streaking) is good for obtaining well-isolated colonies. Incubate the plate only until good colonies have formed. Do not leave the culture in the incubator beyond that time, or the cells will die on the plate. Store the plate in the refrigerator. It will keep for about a month. For class use, it is best to streak a fresh plate 2-3 days before the strain will be needed for class. Use the fresh plate as the source of cultures for the class. For broth cultures for a class, it is best to grow a larger volume culture in a flask (if possible, with shaking) overnight and then dispense aliquots of the master culture into sterile test tubes using a sterile pipette. -

Pouring Plates from Prepared Bottled Media
Pouring Plates from Prepared Bottled Media Primary Hazard Warning Never purchase living specimens without having a disposition strategy in place. When pouring bottles, agar is HOT! Burning can occur. Always handle hot agar bottles with heat-protective gloves. For added protection wear latex or nitrile gloves when working with bacteria, and always wash hands before and after with hot water and soap. Availability Agar is available for purchase year round. Information • Storage: Bottled agar can be stored at room temperature for about six months unless otherwise specified. Never put agar in the freezer. It will cause the agar to breakdown and become unusable. To prevent contamination keep all bottles and Petri dishes sealed until ready to use. • Pouring Plates • Materials Needed: • Draft-free enclosure or Laminar flow hood • 70% isopropyl alcohol • Petri dishes • Microwave or hot water bath or autoclave 1. Melt the agar using one of the following methods: a) Autoclave: Loosen the cap on the agar bottle and autoclave the bottle at 15 psi for five minutes. While wearing heat-protective gloves, carefully remove the hot bottle and let it cool to between 75–55°C before pouring. This takes approximately 15 minutes. b)Water Bath: Loosen the cap on the agar bottle and place it into a water bath. Water temperature should remain at around 100°C. Leave it in the water bath until the agar is completely melted. While wearing heat- protective gloves, carefully remove the hot bottle and let it cool to between 75–55°C before pouring. c) Microwave: Loosen the cap on the agar bottle before microwaving. -

Technical Guidance for Microbiology Incubators
DRAFT – FOR DISCUSSION PURPOSES ONLY DEPARTMENT OF ENVIRONMENTAL PROTECTION Laboratory Accreditation Program Document Number: # Title: Microbiology Incubator Guidance Effective Date: Date Authority: 27 Pa.C.S. §§ 4101 – 4113 (relating to environmental laboratory accreditation), Environmental Laboratory Accreditation Regulations, 25 Pa. Code Chapter 252 Policy: It is the policy of the Department of Environmental Protection (DEP) to provide laboratory management personnel with the information necessary to either obtain or maintain accreditation to perform and report environmental analyses in Pennsylvania. Purpose: This technical guidance will provide laboratory management with the tools to develop procedures for maintenance and verification of incubation units used for microbiological testing that meet the requirements for laboratory accreditation. Applicability: This guidance will apply to all laboratories desiring to obtain and/or maintain accreditation under 25 Pa. Code Chapter 252. Disclaimer: The policies and procedures outlined in this guidance document are intended to supplement existing requirements. Nothing in the policies or procedures will affect regulatory requirements. The policies and procedures herein are not an adjudication or a regulation. There is no intent on the part of the Department to give these rules that weight or deference. This document establishes the framework within which DEP will exercise its administrative discretion in the future. DEP reserves the discretion to deviate from this policy statement if circumstances -

Laboratory Exercises in Microbiology: Discovering the Unseen World Through Hands-On Investigation
City University of New York (CUNY) CUNY Academic Works Open Educational Resources Queensborough Community College 2016 Laboratory Exercises in Microbiology: Discovering the Unseen World Through Hands-On Investigation Joan Petersen CUNY Queensborough Community College Susan McLaughlin CUNY Queensborough Community College How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/qb_oers/16 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] Laboratory Exercises in Microbiology: Discovering the Unseen World through Hands-On Investigation By Dr. Susan McLaughlin & Dr. Joan Petersen Queensborough Community College Laboratory Exercises in Microbiology: Discovering the Unseen World through Hands-On Investigation Table of Contents Preface………………………………………………………………………………………i Acknowledgments…………………………………………………………………………..ii Microbiology Lab Safety Instructions…………………………………………………...... iii Lab 1. Introduction to Microscopy and Diversity of Cell Types……………………......... 1 Lab 2. Introduction to Aseptic Techniques and Growth Media………………………...... 19 Lab 3. Preparation of Bacterial Smears and Introduction to Staining…………………...... 37 Lab 4. Acid fast and Endospore Staining……………………………………………......... 49 Lab 5. Metabolic Activities of Bacteria…………………………………………….…....... 59 Lab 6. Dichotomous Keys……………………………………………………………......... 77 Lab 7. The Effect of Physical Factors on Microbial Growth……………………………... 85 Lab 8. Chemical Control of Microbial Growth—Disinfectants and Antibiotics…………. 99 Lab 9. The Microbiology of Milk and Food………………………………………………. 111 Lab 10. The Eukaryotes………………………………………………………………........ 123 Lab 11. Clinical Microbiology I; Anaerobic pathogens; Vectors of Infectious Disease….. 141 Lab 12. Clinical Microbiology II—Immunology and the Biolog System………………… 153 Lab 13. Putting it all Together: Case Studies in Microbiology…………………………… 163 Appendix I. -

Microlab® STAR™
Microlab ® STAR™ Microlab ® STAR ™ AUTOMATED WORKFLOW SOLUTIONS CENTERED AROUND YOUR ASSAY The STAR combines Hamilton's patented pipetting technology including precise lock-and-key tip attachment, unrivaled liquid level detection, and comprehensive volume ranges to create flexible liquid handling workstations. Available in three base platform sizes, the STAR portfolio incorporates countless options to automate your workflows. Hamilton Robotics has also partnered with top leaders in the biotechnology industry to provide Standard Solutions based on commonly automated applications. Offering ready-to-start protocols for a variety of applications such as NGS, ELISA, and forensic assays, our Standard Solutions provide a faster way to automate your processes. 2 1 PATENTED TECHNOLOGY The STAR utilizes Hamilton’s proprietary Compressed O-Ring Expansion (CO-RE®) technology. CO-RE minimizes the production of aerosols and allows disposable tips or washable, steel needles to be used on channels in the same run. 2 MULTI-FUNCTIONAL ARM Our technology offers high pipetting accuracy and precision, from sub-microliter to large volumes, using Independent Channels and/or the Multi-Probe Head (MPH). Labware transportation is possible with the iSWAP® or CO-RE Grippers. The STAR can incorporate a camera, tube transportation, and other channel tools on a single arm. Comprehensive pipetting range: ■■■0.5 μL to 1 mL using the 1 mL Independent Channel ■■■50 μL to 5 mL using the 5 mL Independent Channel ■■■1 μL to 1 mL using the CO-RE 96 MPH ■■■0.1 μL to 50 μL using the CO-RE 384 MPH 3 FLEXIBLE SETUP The high-capacity deck is customized specific to your workflow, accommodating a wide range of labware and automated devices that can easily be exchanged to support multiple assays on one platform. -

Thermo Scientific Solutions Automated Liquid Handling, Detection Model Cat
THERMO SCIENTIFIC AUTOMATED LIQUID HANDLING, DETECTION AND SAMPLE PURIFICATION SOLUTIONS Versette™ RapidStak™ Also available: 96-and 384-channel Automated Microplate • A complete range of 96- and 384-well solid and strip microplates. Automated Liquid Handler Stacker • A complete line of sample storage tubes and equipment. • Total volume range 0.5-300 µl • Works seamlessly with • A complete range of Thermo Scientific manual and electronic • 96- and 384-channel interchangeable the entire Multidrop line of single and multichannel pipettes and tips. pipetting heads dispensers • Laboratory automation solutions for microplate instrument systems. • Compatible with D.A.R.T.sTM tips with • Schedule and automate any unique sealing properties two instruments with the • 6-position stage with compact dual level Thermo ScientificTM PolaraTM RS deck structure software For additional information contact your ™ • User-friendly programming options • Capacity from 30 to 150 plates Thermo Fisher Scientific sales representative or visit: with onboard touch screen or Thermo • Quick and easy setup www.thermoscientific.com/kingfisherinfo ScientificTM ControlMateTM PC software Model Cat. No. www.thermoscientific.com/versette RapidStak F01350 www.thermoscientific.com/multidrop RapidStak 2x F01351 Model Cat. No. www.thermoscientific.com/platereaders 30-Plate Stak Versette F01436 www.thermoscientific.com/wellwash Reference Guide Versette base unit 650-01-BS 50-Plate Stak F01437 www.thermoscientifc.com/ELISAsolutions 96- and 384-channel pipetting module for use with 650-02-NTC Polara RS Inquire 96- and 384-channel pipetting head 6-position stage 650-03-SPS Versette Pipetting Heads Orbitor RS™ 96-channel air displacement pipetting head. 650-06-9630 Volume 0.5-30 µl Automated Microplate Mover • Dedicated plate mover providing 96-channel air displacement pipetting head.