Microlab® STAR™
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Exploring Small-Scale Chemostats to Scale up Microbial Processes: 3-Hydroxypropionic Acid Production in S
Lawrence Berkeley National Laboratory Recent Work Title Exploring small-scale chemostats to scale up microbial processes: 3-hydroxypropionic acid production in S. cerevisiae. Permalink https://escholarship.org/uc/item/43h8866j Journal Microbial cell factories, 18(1) ISSN 1475-2859 Authors Lis, Alicia V Schneider, Konstantin Weber, Jost et al. Publication Date 2019-03-11 DOI 10.1186/s12934-019-1101-5 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Lis et al. Microb Cell Fact (2019) 18:50 https://doi.org/10.1186/s12934-019-1101-5 Microbial Cell Factories RESEARCH Open Access Exploring small-scale chemostats to scale up microbial processes: 3-hydroxypropionic acid production in S. cerevisiae Alicia V. Lis1, Konstantin Schneider1,2, Jost Weber1,3, Jay D. Keasling1,4,5,6,7, Michael Krogh Jensen1 and Tobias Klein1,2* Abstract Background: The physiological characterization of microorganisms provides valuable information for bioprocess development. Chemostat cultivations are a powerful tool for this purpose, as they allow defned changes to one single parameter at a time, which is most commonly the growth rate. The subsequent establishment of a steady state then permits constant variables enabling the acquisition of reproducible data sets for comparing microbial perfor- mance under diferent conditions. We performed physiological characterizations of a 3-hydroxypropionic acid (3-HP) producing Saccharomyces cerevisiae strain in a miniaturized and parallelized chemostat cultivation system. The physi- ological conditions under investigation were various growth rates controlled by diferent nutrient limitations (C, N, P). Based on the cultivation parameters obtained subsequent fed-batch cultivations were designed. Results: We report technical advancements of a small-scale chemostat cultivation system and its applicability for reliable strain screening under diferent physiological conditions, i.e. -

Microscope Compatible Cell-Culture Incubator
Microscope Compatible Cell-Culture Incubator BME 400 December 14th, 2016 Client: Dr. John Puccinelli, UW-Madison Dept. of Biomedical Engineering Advisor: Dr. Mitchell Tyler, UW-Madison Dept. of Biomedical Engineering Team Members: Trevor Zarecki - Team Leader Jenny Westlund - Communicator Peter Hartig - BPAG Jack McGinnity - BWIG Steve Gock - BSAC Abstract Live cell imaging experiments are difficult to perform over long periods of time on normal lab microscopes without incubation capabilities. Current commercial microscope-stage incubators are expensive, not compatible with multiple microscopes and ineffective at evenly controlling the environment. The client desires an inexpensive incubation chamber compatible with cell microscopy that is capable of maintaining desired temperature, CO2, and humidity evenly throughout the chamber. The device should not alter image quality, and should be accessible for changing media or cell culture dishes. An initial prototype has been developed that involves a small, cohesive system to regulate temperature, CO2, and humidity. Testing of the current prototype has demonstrated regulation of these three systems, through an automated feedback system capable of maintaining the system near a physiological set point. Further development of the design will ensure that it performs efficiently according to all specifications, and ultimately help bridge the gap in the market between high-cost, functional incubation systems and cheaper, less effective designs. 1 Table of Contents Introduction 3 Motivation 3 Existing -

Effective Contamination Control with CO2 Incubators
WHITE PAPER No. 30 Effective Contamination Control with CO2 Incubators Ines Kristina Hartmann¹, James Jarvis² ¹Eppendorf AG, ²Eppendorf, Inc. Executive Summary CO2 incubators provide an optimal cell growth environ- ment by maintaining a humidified atmosphere with temperature and carbon dioxide control. These conditions not only promote cell growth, but also the growth of contaminants, like bacteria, yeast, molds and other fungi. The contamination-reducing features of an incubator’s functional design and the effectiveness of its self-decon- tamination system must be considered in choosing an instrument. In this paper, we compare various strategies for preventing contamination in CO2 incubators, from the functional design of the device to self-disinfection programs. We also give some useful tips to prevent contamination when using CO2 incubators. Introduction Sources of contamination How do contaminants get into a CO2 incubator? Contamination is a major cause of frustration when culturing The CO2 incubator may become an indirect source of con- cells. There are many sources of contamination, either direct tamination. Unlike a biological safety cabinet, an incubator or indirect. Direct sources are contaminated reagents, media cannot prevent the influx of airborne contaminants, as the or seed culture. Media and reagents from reputable suppliers door must be opened during routine use. The incubator are rarely delivered contaminated nowadays. New cell lines chamber can also be contaminated by carelessness in aseptic can introduce contaminants into the lab, especially when techniques, and unnoticed splashes from cell culture vessels. they are given from lab to lab. They should be quarantined Some CO2 incubators use a HEPA filter to remove microor- before culturing with the rest of the cells. -

Microbiology Laboratory Exercises Third Edition 2020
MICROBIOLOGY Laboratory Exercises Third Edition Keddis & Rauschenbach 2020 Photo Credits (in order of contribution): Diane Davis, Ines Rauschenbach & Ramaydalis Keddis Acknowledgements: Many thanks to those in the Department of Biochemistry and Microbiology, Rutgers University, who have through the years inspired our enthusiasm for the science and teaching of microbiology, with special thanks to Diane Davis, Douglas Eveleigh and Max Häggblom. Safety: The experiments included in this manual have been deemed safe by the authors when all necessary safety precautions are met. The authors recommend maintaining biosafety level 2 in the laboratory setting and using risk level 1 organisms for all exercises. License: This work is licensed under a Creative Commons Attribution- NonCommercial-NoDerivatives 4.0 International License Microbiology Laboratory Exercises Third Edition 2020 Ramaydalis Keddis, Ph.D. Ines Rauschenbach, Ph.D. Department of Biochemistry and Microbiology Rutgers, The State University of New Jersey CONTENTS PAGE Introduction Schedule ii Best Laboratory Practices Iii Working in a Microbiology Laboratory iv Exercises Preparation of a Culture Medium 1 Culturing and Handling Microorganisms 3 Isolation of a Pure Culture 5 Counting Bacterial Populations 8 Controlling Microorganisms 10 Disinfectants 10 Antimicrobial Agents: Susceptibility Testing 12 Hand Washing 14 The Lethal Effects of Ultraviolet Light 15 Selection of Fungi from Air 17 Microscopy 21 Morphology and Staining of Bacteria 26 Microbial Metabolism 30 Enzyme Assay 32 Metabolic -
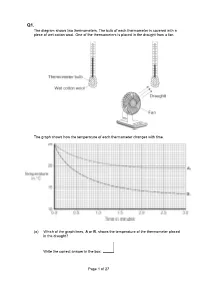
Page 1 of 27 the Diagram Shows Two Thermometers. the Bulb of Each
Q1. The diagram shows two thermometers. The bulb of each thermometer is covered with a piece of wet cotton wool. One of the thermometers is placed in the draught from a fan. The graph shows how the temperature of each thermometer changes with time. (a) Which of the graph lines, A or B, shows the temperature of the thermometer placed in the draught? Write the correct answer in the box. Page 1 of 27 Explain, in terms of evaporation, the reason for your answer. ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ (3) (b) A wet towel spread out and hung outside on a day without wind dries faster than an identical wet towel left rolled up in a plastic bag. Explain why. ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ (2) (Total 5 marks) Q2. The picture shows a person taking a hot shower. (a) When a person uses the shower the mirror gets misty. Why? ___________________________________________________________________ ___________________________________________________________________ -

Pouring Plates from Prepared Bottled Media
Pouring Plates from Prepared Bottled Media Primary Hazard Warning Never purchase living specimens without having a disposition strategy in place. When pouring bottles, agar is HOT! Burning can occur. Always handle hot agar bottles with heat-protective gloves. For added protection wear latex or nitrile gloves when working with bacteria, and always wash hands before and after with hot water and soap. Availability Agar is available for purchase year round. Information • Storage: Bottled agar can be stored at room temperature for about six months unless otherwise specified. Never put agar in the freezer. It will cause the agar to breakdown and become unusable. To prevent contamination keep all bottles and Petri dishes sealed until ready to use. • Pouring Plates • Materials Needed: • Draft-free enclosure or Laminar flow hood • 70% isopropyl alcohol • Petri dishes • Microwave or hot water bath or autoclave 1. Melt the agar using one of the following methods: a) Autoclave: Loosen the cap on the agar bottle and autoclave the bottle at 15 psi for five minutes. While wearing heat-protective gloves, carefully remove the hot bottle and let it cool to between 75–55°C before pouring. This takes approximately 15 minutes. b)Water Bath: Loosen the cap on the agar bottle and place it into a water bath. Water temperature should remain at around 100°C. Leave it in the water bath until the agar is completely melted. While wearing heat- protective gloves, carefully remove the hot bottle and let it cool to between 75–55°C before pouring. c) Microwave: Loosen the cap on the agar bottle before microwaving. -

Technical Guidance for Microbiology Incubators
DRAFT – FOR DISCUSSION PURPOSES ONLY DEPARTMENT OF ENVIRONMENTAL PROTECTION Laboratory Accreditation Program Document Number: # Title: Microbiology Incubator Guidance Effective Date: Date Authority: 27 Pa.C.S. §§ 4101 – 4113 (relating to environmental laboratory accreditation), Environmental Laboratory Accreditation Regulations, 25 Pa. Code Chapter 252 Policy: It is the policy of the Department of Environmental Protection (DEP) to provide laboratory management personnel with the information necessary to either obtain or maintain accreditation to perform and report environmental analyses in Pennsylvania. Purpose: This technical guidance will provide laboratory management with the tools to develop procedures for maintenance and verification of incubation units used for microbiological testing that meet the requirements for laboratory accreditation. Applicability: This guidance will apply to all laboratories desiring to obtain and/or maintain accreditation under 25 Pa. Code Chapter 252. Disclaimer: The policies and procedures outlined in this guidance document are intended to supplement existing requirements. Nothing in the policies or procedures will affect regulatory requirements. The policies and procedures herein are not an adjudication or a regulation. There is no intent on the part of the Department to give these rules that weight or deference. This document establishes the framework within which DEP will exercise its administrative discretion in the future. DEP reserves the discretion to deviate from this policy statement if circumstances -

Orbital Shaker Benchmarks: Best Practices for Use and Maintenance
APPLICATION NOTE Orbital shaker benchmarks: best practices for use and maintenance Authors: Mary Kay Bates, Senior Global Cell Culture Specialist and Sara Livingston, Global Product Manager, Thermo Fisher Scientific Key words: Orbital shaker, HEPA filter, cleaning, orbit diameter INTRODUCTION Most life science labs have an orbital shaker—often more than one— because these versatile workhorses are used in many applications including chemistry, biochemistry, molecular biology, microbiology, environmental and food sciences, clinical diagnostics, and eukaryotic Figure 1. Orbital shakers are available in a wide variety of sizes and formats. cell culture. Usage extends from basic research all the way up to maintenance schedule. In all cases, for before discarding any packing bioproduction. Shakers come in the manufacturer’s recommendations materials; some small parts can be a wide range of sizes and formats for each model take precedence and accidentally discarded. (Figure 1), including open air, heated, should be followed. or refrigerated. Choosing among Recognize that orbital shakers, these many options can be confusing, INSTALLATION regardless of size, can be heavier than and your application may require a Upon delivery of your new shaker, you may think. Take care when lifting specific shaker style1. inspect the shipping carton and the shaker, ensure you have at least ask the carrier to specify and sign one other person to help you, and that Regardless of the models used, this for any damage on your delivery the shelf or bench designated to hold application note contains valuable receipt, to ensure that any damage the shaker will be strong enough. information which applies to all to the components can be properly orbital shakers. -

Thermo Scientific Solutions Automated Liquid Handling, Detection Model Cat
THERMO SCIENTIFIC AUTOMATED LIQUID HANDLING, DETECTION AND SAMPLE PURIFICATION SOLUTIONS Versette™ RapidStak™ Also available: 96-and 384-channel Automated Microplate • A complete range of 96- and 384-well solid and strip microplates. Automated Liquid Handler Stacker • A complete line of sample storage tubes and equipment. • Total volume range 0.5-300 µl • Works seamlessly with • A complete range of Thermo Scientific manual and electronic • 96- and 384-channel interchangeable the entire Multidrop line of single and multichannel pipettes and tips. pipetting heads dispensers • Laboratory automation solutions for microplate instrument systems. • Compatible with D.A.R.T.sTM tips with • Schedule and automate any unique sealing properties two instruments with the • 6-position stage with compact dual level Thermo ScientificTM PolaraTM RS deck structure software For additional information contact your ™ • User-friendly programming options • Capacity from 30 to 150 plates Thermo Fisher Scientific sales representative or visit: with onboard touch screen or Thermo • Quick and easy setup www.thermoscientific.com/kingfisherinfo ScientificTM ControlMateTM PC software Model Cat. No. www.thermoscientific.com/versette RapidStak F01350 www.thermoscientific.com/multidrop RapidStak 2x F01351 Model Cat. No. www.thermoscientific.com/platereaders 30-Plate Stak Versette F01436 www.thermoscientific.com/wellwash Reference Guide Versette base unit 650-01-BS 50-Plate Stak F01437 www.thermoscientifc.com/ELISAsolutions 96- and 384-channel pipetting module for use with 650-02-NTC Polara RS Inquire 96- and 384-channel pipetting head 6-position stage 650-03-SPS Versette Pipetting Heads Orbitor RS™ 96-channel air displacement pipetting head. 650-06-9630 Volume 0.5-30 µl Automated Microplate Mover • Dedicated plate mover providing 96-channel air displacement pipetting head. -

Kjeldahl Distillation Unit
Terms of Business v There are frequent fluctuations in the cost of input. So we may not be able to give prior intimation regarding changes in the price structure. v Packing, Forwarding, Freight and Insurance will be Charged Extra. v Sales Tax and Excise Duty will be extra as per Central or State Government Rules. v Every possible care is taken in packing, but it is difficult to undertake responsibility of loss, breakage or damage during transit. v R/R and our bill be sent though V. P. P. or any scheduled bank. New customers are requested to advance 25% of the estimated value. v While placing the order, please give clear despatch instructions indicating C. S. T. Number, Name of Railway Station, Your Banker and Our Catalogue Number. v All problems shall be solved amicably subject to Ambala Jurisdiction. GUPTA SCIENTIFIC INDUSTRIES Ambala Cantt. THIS CANCELS OUR ALL PREVIOUS PRICE LISTS PPPrice List 2017-18 CATALOGUE CONTENTS Product Range Pages Interchangeable Standard Joints, Adapters, Stirrers, Condensers, 1 to 49 Laboratory Flasks, Columns, Separating Funnels, Assemblies, Glasswares Water Distillation, Essential Oil, Soxhlet Apparatus. Volumetric Burettes, Pipettes, Micro Pipettes, Measuring Cylinders, 50 to 74 Glasswares Measuring Flasks, Culture Tubes, Centrifuge Tube & Test Tubes. Sintered Sintered Crucibles, Funnels, Chromatography Columns and 75 to 84 Glasswares Filter Assembly. Gas Estimation Impinger, Gas Burettes, Tubes, Gas Wash Bottles, 85 to 90 Apparatus Oxygen Purity and Orsat Apparatus. Special Semi Micro Ware, Clinical Laboratory Apparatus, 91 to 97 Glasswares Electrodes and Milk Testing Apparatus. Supplementary Stopcocks, Rotaflow Screw Type Stopcocks, Teflon Key 98 to 123 Glasswares Stopcocks, Manometers, Weighing Bottles, S. -

Evobot: an Open-Source, Modular, Liquid Handling Robot for Scientific Experiments
applied sciences Article EvoBot: An Open-Source, Modular, Liquid Handling Robot for Scientific Experiments Andres Faiña 1,* , Brian Nejati 2 and Kasper Stoy 1 1 Robots, Evolution and Art Lab (REAL), Department of Computer Science, IT University of Copenhagen, 2300 Copenhagen, Denmark; [email protected] 2 Department of Mechanical and Industrial Engineering, University of Toronto, Toronto, ON M5S 1A1, Canada; His work was carried out while he was employed at ITU; [email protected] * Correspondence: [email protected]; Tel.: +45-7218-5258 Received: 13 October 2019; Accepted: 17 January 2020; Published: 23 January 2020 Featured Application: Our modular liquid handling robot can be applied to automate scientific experiments in research labs, where tasks change often, and to carry out new kinds of experiments that cannot be done manually. Abstract: Commercial liquid handling robots are rarely appropriate when tasks change often, which is the case in the early stages of biochemical research. In order to address it, we have developed EvoBot, a liquid handling robot, which is open-source and employs a modular design. The combination of an open-source and a modular design is particularly powerful because functionality is divided into modules with simple, well-defined interfaces, hence customisation of modules is possible without detailed knowledge of the entire system. Furthermore, the modular design allows end-users to only produce and assemble the modules that are relevant for their specific application. Hence, time and money are not wasted on functionality that is not needed. Finally, modules can easily be reused. In this paper, we describe the EvoBot modular design and through scientific experiments such as basic liquid handling, nurturing of microbial fuel cells, and droplet chemotaxis experiments document how functionality is increased one module at a time with a significant amount of reuse. -

List of Equipments in the Department (Chemical Engineering) Mechanical
List of Equipments in the Department (Chemical Engineering) Mechanical Operation lab Fluid Mechanics Lab Heat transfer Lab Mass Transfer lab • Cyclone Separator • Reynold’s Apparatus • Double Pipe Heat Exchanger • Tray Dryer • Plate & Frame Filter Press • Bernoulli’s Theorem Apparatus • Shell & Tube Heat Exchanger • Sieve Plate Distillation Column • Vibrating Screen • Pitot Tube Apparatus • Vertical Condenser • Liquid-Liquid Extraction • Jaw Crusher • Calibration of Orifice meter, • Computerized Control Shell & • Adsorption of CO 2 • Ball Mill Venturi meter and Rota meter Tube Heat Exchanger • Steam Distillation • Roll Crusher • Coefficient of Discharge of • Thermal Conductivity of Metal • Bubble Cap Distillation Column • Rotary Vacuum filter Orifice and Mouthpiece Bar • Cooling Tower • Fluidized Bed • Film & Drop Wise Condensation • Diffusivity Apparatus • Single Effect Evaporator • Fluidized Bed Dryer • Simple Steam Distillation • Simple Distillation • VLE Apparatus • Equilibrium Flash Distillation • Humidification & Dehumidification • Refractometer Process Control Lab Chemical Reaction Engg. Lab Fuel Combustion Energy Technology Lab Environmental Lab • Process Training Simulator with • Plug Flow Reactor • Flash & Fire Point Apparatus • BOD Incubator Modules (Software) • Isothermal Batch Reactor • Aniline Point Apparatus • pH Meter • Pressure Control System • Single Tube Packed Bed Reactor • Orsat Gas Analysis Apparatus • Sedimentation Apparatus • Flow Control System • RTD in CSTR / Mixed flow • Redwood Viscometer • UV-VIS Spectrophotometer • pH control System Reactor • Bomb Calorimeter • DO Meter • Real Time Simulator Trainer • RTD in Tubular Reactor • Smoke Point Apparatus • Digital Conductivity Meter • Level Control System • Adiabatic Batch Reactor • Temperature Control System • Distillation Column • Two Tank Interacting system • Two Tank Non-Interacting System • CSTR Control System Research Equipments • UV-Spectrophotometer • Gas Chomatography .