Pure Culture Techniques
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tryptose Blood Agar Base
Tryptose Blood Agar Base Intended Use Principles of the Procedure Tryptose Blood Agar Base is used with blood in isolating, Tryptose is the source of nitrogen, carbon and amino acids in cultivating and determining the hemolytic reactions of fastidi- Tryptose Blood Agar Base. Beef extract provides additional ous microorganisms. nitrogen. Sodium chloride maintains osmotic balance. Agar is the solidifying agent. Summary and Explanation Investigations of the nutritive properties of tryptose demon- Supplementation with 5-10% blood provides additional growth strated that culture media prepared with this peptone were factors for fastidious microorganisms and is used to determine superior to the meat infusion peptone media previously used hemolytic patterns of bacteria. for the cultivation of Brucella, streptococci, pneumococci, me- Formula ningococci and other fastidious bacteria. Casman1,2 reported Difco™ Tryptose Blood Agar Base that a medium consisting of 2% tryptose, 0.3% beef extract, Approximate Formula* Per Liter 0.5% NaCl, 1.5% agar and 0.03% dextrose equaled fresh beef Tryptose .................................................................... 10.0 g infusion base with respect to growth of organisms. The small Beef Extract ................................................................. 3.0 g amount of carbohydrate was noted to interfere with hemolytic Sodium Chloride ......................................................... 5.0 g Agar ......................................................................... 15.0 g reactions, unless the medium was incubated in an atmosphere *Adjusted and/or supplemented as required to meet performance criteria. of carbon dioxide. Tryptose Blood Agar Base is a nutritious infusion-free basal Directions for Preparation from medium typically supplemented with 5-10% sheep, rabbit or Dehydrated Product horse blood for use in isolating, cultivating and determining 1. Suspend 33 g of the powder in 1 L of purified water. -

Microbiology Laboratory Exercises Third Edition 2020
MICROBIOLOGY Laboratory Exercises Third Edition Keddis & Rauschenbach 2020 Photo Credits (in order of contribution): Diane Davis, Ines Rauschenbach & Ramaydalis Keddis Acknowledgements: Many thanks to those in the Department of Biochemistry and Microbiology, Rutgers University, who have through the years inspired our enthusiasm for the science and teaching of microbiology, with special thanks to Diane Davis, Douglas Eveleigh and Max Häggblom. Safety: The experiments included in this manual have been deemed safe by the authors when all necessary safety precautions are met. The authors recommend maintaining biosafety level 2 in the laboratory setting and using risk level 1 organisms for all exercises. License: This work is licensed under a Creative Commons Attribution- NonCommercial-NoDerivatives 4.0 International License Microbiology Laboratory Exercises Third Edition 2020 Ramaydalis Keddis, Ph.D. Ines Rauschenbach, Ph.D. Department of Biochemistry and Microbiology Rutgers, The State University of New Jersey CONTENTS PAGE Introduction Schedule ii Best Laboratory Practices Iii Working in a Microbiology Laboratory iv Exercises Preparation of a Culture Medium 1 Culturing and Handling Microorganisms 3 Isolation of a Pure Culture 5 Counting Bacterial Populations 8 Controlling Microorganisms 10 Disinfectants 10 Antimicrobial Agents: Susceptibility Testing 12 Hand Washing 14 The Lethal Effects of Ultraviolet Light 15 Selection of Fungi from Air 17 Microscopy 21 Morphology and Staining of Bacteria 26 Microbial Metabolism 30 Enzyme Assay 32 Metabolic -
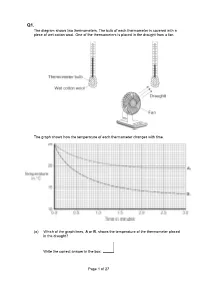
Page 1 of 27 the Diagram Shows Two Thermometers. the Bulb of Each
Q1. The diagram shows two thermometers. The bulb of each thermometer is covered with a piece of wet cotton wool. One of the thermometers is placed in the draught from a fan. The graph shows how the temperature of each thermometer changes with time. (a) Which of the graph lines, A or B, shows the temperature of the thermometer placed in the draught? Write the correct answer in the box. Page 1 of 27 Explain, in terms of evaporation, the reason for your answer. ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ (3) (b) A wet towel spread out and hung outside on a day without wind dries faster than an identical wet towel left rolled up in a plastic bag. Explain why. ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ (2) (Total 5 marks) Q2. The picture shows a person taking a hot shower. (a) When a person uses the shower the mirror gets misty. Why? ___________________________________________________________________ ___________________________________________________________________ -

The Genus Staphylococcus 19 171
43038_CH19_0171.qxd 1/3/07 3:53 PM Page 171 THE GENUS STAPHYLOCOCCUS 19 171 The Genus 19 Staphylococcus embers of the genus Staphylococcus are gram-positive spherical organisms about 1 micrometer in diameter. They M occur singly, in pairs, and in irregular clusters, and form yel- low, orange, or white colonies on agar media. They are salt tolerant and grow on ordinary bacteriological media as well as on the selective media used in this exercise. Up to three species of Staphylococcus are studied in this exercise. Cer- tain strains of Staphylococcus aureus are the cause of food poisoning and toxic shock syndrome. They also are the cause of boils and carbuncles. A second species, S. epidermidis, usually is a saprobe of the skin that is rarely involved in human infection. The third species, S. saprophyticus, is an opportunistic species that may cause urinary tract infections in women of childbearing years. In this exercise, staphylococcal species will be isolated from the body’s environment and their properties examined. A. Isolation of Staphylococci Species of Staphylococcus are tolerant to salt and, therefore, they can be PURPOSE: to isolate and selected out from a mixture of bacteria in a high-salt medium. In addition, identify staphylococcal species from the nasal cavity S. aureus ferments mannitol, an alcoholic derivative of the hexose mannose, and other environments. while S. epidermidis and S. saprophyticus do not. Therefore, if the differential medium contains mannitol, the two species may be differentiated from one another. In this section, we will use mannitol salt agar, a medium that is both selective and differential. -

Pouring Plates from Prepared Bottled Media
Pouring Plates from Prepared Bottled Media Primary Hazard Warning Never purchase living specimens without having a disposition strategy in place. When pouring bottles, agar is HOT! Burning can occur. Always handle hot agar bottles with heat-protective gloves. For added protection wear latex or nitrile gloves when working with bacteria, and always wash hands before and after with hot water and soap. Availability Agar is available for purchase year round. Information • Storage: Bottled agar can be stored at room temperature for about six months unless otherwise specified. Never put agar in the freezer. It will cause the agar to breakdown and become unusable. To prevent contamination keep all bottles and Petri dishes sealed until ready to use. • Pouring Plates • Materials Needed: • Draft-free enclosure or Laminar flow hood • 70% isopropyl alcohol • Petri dishes • Microwave or hot water bath or autoclave 1. Melt the agar using one of the following methods: a) Autoclave: Loosen the cap on the agar bottle and autoclave the bottle at 15 psi for five minutes. While wearing heat-protective gloves, carefully remove the hot bottle and let it cool to between 75–55°C before pouring. This takes approximately 15 minutes. b)Water Bath: Loosen the cap on the agar bottle and place it into a water bath. Water temperature should remain at around 100°C. Leave it in the water bath until the agar is completely melted. While wearing heat- protective gloves, carefully remove the hot bottle and let it cool to between 75–55°C before pouring. c) Microwave: Loosen the cap on the agar bottle before microwaving. -

Laboratory Exercises in Microbiology: Discovering the Unseen World Through Hands-On Investigation
City University of New York (CUNY) CUNY Academic Works Open Educational Resources Queensborough Community College 2016 Laboratory Exercises in Microbiology: Discovering the Unseen World Through Hands-On Investigation Joan Petersen CUNY Queensborough Community College Susan McLaughlin CUNY Queensborough Community College How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/qb_oers/16 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] Laboratory Exercises in Microbiology: Discovering the Unseen World through Hands-On Investigation By Dr. Susan McLaughlin & Dr. Joan Petersen Queensborough Community College Laboratory Exercises in Microbiology: Discovering the Unseen World through Hands-On Investigation Table of Contents Preface………………………………………………………………………………………i Acknowledgments…………………………………………………………………………..ii Microbiology Lab Safety Instructions…………………………………………………...... iii Lab 1. Introduction to Microscopy and Diversity of Cell Types……………………......... 1 Lab 2. Introduction to Aseptic Techniques and Growth Media………………………...... 19 Lab 3. Preparation of Bacterial Smears and Introduction to Staining…………………...... 37 Lab 4. Acid fast and Endospore Staining……………………………………………......... 49 Lab 5. Metabolic Activities of Bacteria…………………………………………….…....... 59 Lab 6. Dichotomous Keys……………………………………………………………......... 77 Lab 7. The Effect of Physical Factors on Microbial Growth……………………………... 85 Lab 8. Chemical Control of Microbial Growth—Disinfectants and Antibiotics…………. 99 Lab 9. The Microbiology of Milk and Food………………………………………………. 111 Lab 10. The Eukaryotes………………………………………………………………........ 123 Lab 11. Clinical Microbiology I; Anaerobic pathogens; Vectors of Infectious Disease….. 141 Lab 12. Clinical Microbiology II—Immunology and the Biolog System………………… 153 Lab 13. Putting it all Together: Case Studies in Microbiology…………………………… 163 Appendix I. -

Microlab® STAR™
Microlab ® STAR™ Microlab ® STAR ™ AUTOMATED WORKFLOW SOLUTIONS CENTERED AROUND YOUR ASSAY The STAR combines Hamilton's patented pipetting technology including precise lock-and-key tip attachment, unrivaled liquid level detection, and comprehensive volume ranges to create flexible liquid handling workstations. Available in three base platform sizes, the STAR portfolio incorporates countless options to automate your workflows. Hamilton Robotics has also partnered with top leaders in the biotechnology industry to provide Standard Solutions based on commonly automated applications. Offering ready-to-start protocols for a variety of applications such as NGS, ELISA, and forensic assays, our Standard Solutions provide a faster way to automate your processes. 2 1 PATENTED TECHNOLOGY The STAR utilizes Hamilton’s proprietary Compressed O-Ring Expansion (CO-RE®) technology. CO-RE minimizes the production of aerosols and allows disposable tips or washable, steel needles to be used on channels in the same run. 2 MULTI-FUNCTIONAL ARM Our technology offers high pipetting accuracy and precision, from sub-microliter to large volumes, using Independent Channels and/or the Multi-Probe Head (MPH). Labware transportation is possible with the iSWAP® or CO-RE Grippers. The STAR can incorporate a camera, tube transportation, and other channel tools on a single arm. Comprehensive pipetting range: ■■■0.5 μL to 1 mL using the 1 mL Independent Channel ■■■50 μL to 5 mL using the 5 mL Independent Channel ■■■1 μL to 1 mL using the CO-RE 96 MPH ■■■0.1 μL to 50 μL using the CO-RE 384 MPH 3 FLEXIBLE SETUP The high-capacity deck is customized specific to your workflow, accommodating a wide range of labware and automated devices that can easily be exchanged to support multiple assays on one platform. -

SHEEP BLOOD AGAR - for in Vitro Use Only - Catalogue No
SHEEP BLOOD AGAR - For in vitro use only - Catalogue No. PS58 Our Sheep Blood Agar is a highly nutritious Interpretation of Results medium used for the cultivation and isolation of a variety of microorganisms. Sheep Blood Agar can be used as a primary- Our Sheep Blood Agar is based on the Oxoid plating medium. Primary isolation is performed to formulation; the prepared medium is said to offer separate and isolate organisms present in a sample. improved nutritional value resulting in better growth This separation allows for characterization of and larger colony size as well giving more colony types and may indicate the presence of consistent hemolytic reactions especially among the clinically significant bacteria. When examining streptococci. The base is specifically designed for plates a hand lens or stereoscopic microscope use with sheep blood as horse blood has shown to should be available for examining very small give different and conflicting hemolytic reactions colonies. The different types of colonial when incorporated into other blood agars. morphology appearing on the agar plate should be The nutritional components include pancreatic noted as well as the number of each morphotype digest of casein, neutralized peptone, and yeast present. Hemolysis is also a very useful differential extract, and the addition of sodium chloride characteristic that is best viewed when a bright light provides an osmotically balanced medium for is transmitted from behind the plate. Four different bacterial cells. The addition of 5% defibrinated types of hemolysis can be described: sheep blood allows for the determination of hemolytic reactions, an important differential 1. Alpha-hemolysis ( α) – Partial hemolysis that characteristic. -

LAB 2: Staining and Streaking Protocols for Simple Stain, Gram Stain, Streak Plate Technique and Culture Maintenance
LAB 2: Staining and Streaking Protocols for Simple stain, Gram Stain, Streak Plate Technique and Culture Maintenance Lab 2a: INTRODUCTION To Staining Live specimens are difficult to see with the bright field microscope. The contrast between a cell, which is primarily water, and the background, which is water, is poor. Staining is used to increase contrast and can be employed to provide information about the chemistry of a specimen. Stains, or dyes, are salts in which one of the ions is colored. In a basic stain, the color is in the positively charged ion. In an acidic stain the color is in the negatively charged ion. Bacterial surfaces have a slight negative charge. Thus, there is an affinity between a positively charged color ion and the negatively charged bacterial cell. In the Direct or Positive Staining Procedure a cell takes up a positively charged dye and becomes stained. Methylene blue, crystal violet, and safranin, are all basic dyes. In the Indirect or Negative Staining Procedure, a cell is immersed in a negatively charged dye. As the cell will repel the dye, the cell appears clear in a background of color. Nigrosine is an example of an acidic stain. Staining procedures that use one dye to increase contrast between specimen and background are simple staining procedures. Complex staining procedures employ a series of stains and chemical reagents to increase contrast and reveal information about the specimen. Any staining procedure that allows differentiation of one type of bacterium from another is a differential staining procedure. For all staining procedures, it is first required that cells be fixed to the slide. -

Screening for Asymptomatic Bacteriuria in Adults: an Updated Systematic Review for the U.S
Evidence Synthesis Number 183 Screening for Asymptomatic Bacteriuria in Adults: An Updated Systematic Review for the U.S. Preventive Services Task Force Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 5600 Fishers Lane Rockville, MD 20857 www.ahrq.gov Contract No. HHSA-290-2015-00007-I, Task Order No. 3 Prepared by: Kaiser Permanente Research Affiliates Evidence-based Practice Center Kaiser Permanente Center for Health Research Portland, OR Investigators: Jillian T. Henderson, PhD, MPH Elizabeth M. Webber, MS Sarah I. Bean, MPH AHRQ Publication No. 19-05252-EF-1 September 2019 This report is based on research conducted by the Kaiser Permanente Research Affiliates Evidence-based Practice Center (EPC) under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (HHSA-290-2015-00007-I, Task Order No. 3). The findings and conclusions in this document are those of the authors, who are responsible for its contents, and do not necessarily represent the views of AHRQ. Therefore, no statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. The information in this report is intended to help health care decision makers—patients and clinicians, health system leaders, and policymakers, among others—make well-informed decisions and thereby improve the quality of health care services. This report is not intended to be a substitute for the application of clinical judgment. Anyone who makes decisions concerning the provision of clinical care should consider this report in the same way as any medical reference and in conjunction with all other pertinent information (i.e., in the context of available resources and circumstances presented by individual patients). -

Special Microbiology Practical Week 3. – STREPTOCOCCI Streptococci
Special Microbiology practical week 3. – STREPTOCOCCI Streptococci are Gram-positive, nonmotile, nonsporeforming, catalase-negative cocci that occur in pairs or chains. Older cultures may lose their Gram-positive character. Most streptococci are facultative anaerobes, and some are obligate (strict) anaerobes.Most require enriched media (blood agar). Streptococci are subdivided into groups by antibodies that recognize surface antigens These groups may include one or more species.Serologic grouping is based on antigenic differences in cell wall carbohydrates (groups A to V), in cell wall pili-associated protein, and in the polysaccharide capsule in group B streptococci.Rebecca Lancefield developed the serologic classification scheme in 1933. β-hemolyticstrains possess group- specific cell wall antigens, most of which are carbohydrates. These antigens can be detected by immunologic assays and have been useful for the rapid identification of some important streptococcal pathogens.The most important groupable streptococci are A, B and D. Among the groupable streptococci, infectious disease (particularly pharyngitis) is caused by group A. Group A streptococci have a hyaluronic acid capsule.Streptococcus pneumoniae(a major cause of human pneumonia) and Streptococcus mutansand other so-called viridans streptococci (among the causes of dental caries) do not possess group antigen.Streptococcus pneumoniaehas a polysaccharide capsulethat acts as a virulence factor for the organism,more than 90 different serotypesare known, and these types differ in virulence. Beta Hemolysis Streptococcus pyogenes, or Group A beta-hemolytic Streptococci (GAS), and Streptococcus agalactiae, or Group B beta-hemolytic Streptococci (GBS) blood agar cultures display beta hemolysis. Beta hemolysis (β-hemolysis), called complete hemolysis, is a complete lysisof red blood cells in the media around and under the colonies: the area appears lightened (yellow) and transparent. -

Medical Bacteriology
LECTURE NOTES Degree and Diploma Programs For Environmental Health Students Medical Bacteriology Abilo Tadesse, Meseret Alem University of Gondar In collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education September 2006 Funded under USAID Cooperative Agreement No. 663-A-00-00-0358-00. Produced in collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education. Important Guidelines for Printing and Photocopying Limited permission is granted free of charge to print or photocopy all pages of this publication for educational, not-for-profit use by health care workers, students or faculty. All copies must retain all author credits and copyright notices included in the original document. Under no circumstances is it permissible to sell or distribute on a commercial basis, or to claim authorship of, copies of material reproduced from this publication. ©2006 by Abilo Tadesse, Meseret Alem All rights reserved. Except as expressly provided above, no part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without written permission of the author or authors. This material is intended for educational use only by practicing health care workers or students and faculty in a health care field. PREFACE Text book on Medical Bacteriology for Medical Laboratory Technology students are not available as need, so this lecture note will alleviate the acute shortage of text books and reference materials on medical bacteriology.