What's in Your Petri
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Experiment 5: Molar Volume of a Gas (Mg + Hcl)
1 CHEM 30A EXPERIMENT 5: MOLAR VOLUME OF A GAS (MG + HCL) Learning Outcomes Upon completion of this lab, the student will be able to: 1) Demonstrate a single replacement reaction. 2) Calculate the molar volume of a gas at STP using experimental data. 3) Calculate the molar mass of a metal using experimental data. Introduction Metals that are above hydrogen in the activity series will displace hydrogen from an acid and produce hydrogen gas. Magnesium is an example of a metal that is more active than hydrogen in the activity series. The reaction between magnesium metal and aqueous hydrochloric acid is an example of a single replacement reaction (a type of redox reaction). The chemical equation for this reaction is shown below: Mg(s) + 2HCl(aq) è MgCl2(aq) + H2(g) Equation 1 When the reaction between the metal and the acid is conducted in a eudiometer, the volume of the hydrogen gas produced can be easily determined. In the experiment described below magnesium metal will be reacted with an excess of hydrochloric acid and the volume of hydrogen gas produced at the experimental conditions will be determined. According to Avogadro’s law, the volume of one mole of any gas at Standard Temperature and Pressure (STP = 273 K and 1 atm) is 22.4 L. Two important Gas Laws are required in order to convert the experimentally determined volume of hydrogen gas to that at STP. 1. Dalton’s law of partial pressures. 2. Combined gas law. Dalton’s Law of Partial Pressures According to Dalton’s law of partial pressures in a mixture of non-reacting gases, the total pressure exerted is equal to the sum of the partial pressures of the individual gases. -

Use of Laboratory Equipement
USE OF LABORATORY EQUIPEMENT C. Laboratory Thermometers Most thermometers are based upon the principle that liquids expand when heated. Most common thermometers use mercury or colored alcohol as the liquid. These thermometers are constructed as that a uniform-diameter capillary tube surmounts a liquid reservoir. To calibrate a thermometer, one defines two reference points, normally the freezing point of water (0°C, 32°F) and the boiling point of water (100°C, 212°F) at 1 tam of pressure (1 tam = 760 mm Hg). Once these points are marked on the capillary, its length is then subdivided into uniform divisions called degrees. There are 100° between these two points on the Celsius (°C, or centigrade) scale and 180° between those two points on the Fahrenheit (°F) scale. °F = 1.8 °C + 32 The Thermometer and Its Calibration This section describes the proper technique for checking the accuracy of your thermometer. These measurements will show how measured temperatures (read from thermometer) compare with true temperatures (the boiling and freezing points of water). The freezing point of water is 0°C; the boiling point depends upon atmospheric pressure but at sea level it is 100°C. Option 1: Place approximately 50 mL of ice in a 250-mL beaker and cover the ice with distilled water. Allow about 15 min for the mixture to come to equilibrium and then measure and record the temperature of the mixture. Theoretically, this temperature is 0°C. Option 2: Set up a 250-mL beaker on a wire gauze and iron ring. Fill the beaker about half full with distilled water. -

Laboratory Glassware N Edition No
Laboratory Glassware n Edition No. 2 n Index Introduction 3 Ground joint glassware 13 Volumetric glassware 53 General laboratory glassware 65 Alphabetical index 76 Índice alfabético 77 Index Reference index 78 [email protected] Scharlau has been in the scientific glassware business for over 15 years Until now Scharlab S.L. had limited its sales to the Spanish market. However, now, coinciding with the inauguration of the new workshop next to our warehouse in Sentmenat, we are ready to export our scientific glassware to other countries. Standard and made to order Products for which there is regular demand are produced in larger Scharlau glassware quantities and then stocked for almost immediate supply. Other products are either manufactured directly from glass tubing or are constructed from a number of semi-finished products. Quality Even today, scientific glassblowing remains a highly skilled hand craft and the quality of glassware depends on the skill of each blower. Careful selection of the raw glass ensures that our final products are free from imperfections such as air lines, scratches and stones. You will be able to judge for yourself the workmanship of our glassware products. Safety All our glassware is annealed and made stress free to avoid breakage. Fax: +34 93 715 67 25 Scharlab The Lab Sourcing Group 3 www.scharlab.com Glassware Scharlau glassware is made from borosilicate glass that meets the specifications of the following standards: BS ISO 3585, DIN 12217 Type 3.3 Borosilicate glass ASTM E-438 Type 1 Class A Borosilicate glass US Pharmacopoeia Type 1 Borosilicate glass European Pharmacopoeia Type 1 Glass The typical chemical composition of our borosilicate glass is as follows: O Si 2 81% B2O3 13% Na2O 4% Al2O3 2% Glass is an inorganic substance that on cooling becomes rigid without crystallising and therefore it has no melting point as such. -

PML Brochure
PHYSICAL MEASUREMENT LABORATORY Gauging nature on all scales NIST.GOV/PML PHYSICAL MEASUREMENT LABORATORY (PML) The Physical Measurement Laboratory (PML), a major frequency, electricity, temperature, humidity, pressure operating unit of the National Institute of Standards and vacuum, liquid and gas flow, and electromag- and Technology (NIST), sets the definitive U.S. standards netic, optical, acoustic, and ionizing radiation. PML for nearly every kind of measurement in modern life, collaborates directly with industry, universities, profes- sometimes across more than 20 orders of magni- sional and standards-setting organizations, and other tude. PML is a world leader in the science of physical agencies of government to ensure accuracy and to measurement, devising procedures and tools that make solve problems. It also supports research in many continual progress possible. Exact measurements are fields of urgent national importance, such as manu- absolutely essential to industry, medicine, the research facturing, energy, health, law enforcement and community, and government. All of them depend on homeland security, communications, military defense, PML to develop, maintain, and disseminate the official electronics, the environment, lighting and display, standards for a wide range of quantities, including radiation, remote sensing, space exploration, length, mass, force and shock, acceleration, time and and transportation. IMPACTS ❱ Provides 700 kinds of calibration services ❱ Numerous special testing services NIST.GOV/PML | 2 NIST.GOV/PML -

Chemostat Culture for Yeast Experimental Evolution
Downloaded from http://cshprotocols.cshlp.org/ at Cold Spring Harbor Laboratory Library on August 9, 2017 - Published by Cold Spring Harbor Laboratory Press Protocol Chemostat Culture for Yeast Experimental Evolution Celia Payen and Maitreya J. Dunham1 Department of Genome Sciences, University of Washington, Seattle, Washington 98195 Experimental evolution is one approach used to address a broad range of questions related to evolution and adaptation to strong selection pressures. Experimental evolution of diverse microbial and viral systems has routinely been used to study new traits and behaviors and also to dissect mechanisms of rapid evolution. This protocol describes the practical aspects of experimental evolution with yeast grown in chemostats, including the setup of the experiment and sampling methods as well as best laboratory and record-keeping practices. MATERIALS It is essential that you consult the appropriate Material Safety Data Sheets and your institution’s Environmental Health and Safety Office for proper handling of equipment and hazardous material used in this protocol. Reagents Defined minimal medium appropriate for the experiment For examples, see Protocol: Assembly of a Mini-Chemostat Array (Miller et al. 2015). Ethanol (95%) Glycerol (20% and 50%; sterile) Yeast strain of interest Equipment Agar plates (appropriate for chosen strain) Chemostat array Assemble the apparatus as described in Miller et al. (2013) and Protocol: Assembly of a Mini-Chemostat Array (Miller et al. 2015). Cryo deep-freeze labels Cryogenic vials Culture tubes Cytometer (BD Accuri C6) Glass beads, 4 mm (sterile; for plating yeast cells) Glass cylinder Kimwipes 1Correspondence: [email protected] © 2017 Cold Spring Harbor Laboratory Press Cite this protocol as Cold Spring Harb Protoc; doi:10.1101/pdb.prot089011 559 Downloaded from http://cshprotocols.cshlp.org/ at Cold Spring Harbor Laboratory Library on August 9, 2017 - Published by Cold Spring Harbor Laboratory Press C. -

Microbiology Laboratory Exercises Third Edition 2020
MICROBIOLOGY Laboratory Exercises Third Edition Keddis & Rauschenbach 2020 Photo Credits (in order of contribution): Diane Davis, Ines Rauschenbach & Ramaydalis Keddis Acknowledgements: Many thanks to those in the Department of Biochemistry and Microbiology, Rutgers University, who have through the years inspired our enthusiasm for the science and teaching of microbiology, with special thanks to Diane Davis, Douglas Eveleigh and Max Häggblom. Safety: The experiments included in this manual have been deemed safe by the authors when all necessary safety precautions are met. The authors recommend maintaining biosafety level 2 in the laboratory setting and using risk level 1 organisms for all exercises. License: This work is licensed under a Creative Commons Attribution- NonCommercial-NoDerivatives 4.0 International License Microbiology Laboratory Exercises Third Edition 2020 Ramaydalis Keddis, Ph.D. Ines Rauschenbach, Ph.D. Department of Biochemistry and Microbiology Rutgers, The State University of New Jersey CONTENTS PAGE Introduction Schedule ii Best Laboratory Practices Iii Working in a Microbiology Laboratory iv Exercises Preparation of a Culture Medium 1 Culturing and Handling Microorganisms 3 Isolation of a Pure Culture 5 Counting Bacterial Populations 8 Controlling Microorganisms 10 Disinfectants 10 Antimicrobial Agents: Susceptibility Testing 12 Hand Washing 14 The Lethal Effects of Ultraviolet Light 15 Selection of Fungi from Air 17 Microscopy 21 Morphology and Staining of Bacteria 26 Microbial Metabolism 30 Enzyme Assay 32 Metabolic -
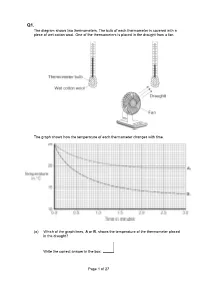
Page 1 of 27 the Diagram Shows Two Thermometers. the Bulb of Each
Q1. The diagram shows two thermometers. The bulb of each thermometer is covered with a piece of wet cotton wool. One of the thermometers is placed in the draught from a fan. The graph shows how the temperature of each thermometer changes with time. (a) Which of the graph lines, A or B, shows the temperature of the thermometer placed in the draught? Write the correct answer in the box. Page 1 of 27 Explain, in terms of evaporation, the reason for your answer. ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ (3) (b) A wet towel spread out and hung outside on a day without wind dries faster than an identical wet towel left rolled up in a plastic bag. Explain why. ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ (2) (Total 5 marks) Q2. The picture shows a person taking a hot shower. (a) When a person uses the shower the mirror gets misty. Why? ___________________________________________________________________ ___________________________________________________________________ -

Ear and Forehead Thermometer
Ear and Forehead Thermometer INSTRUCTION MANUAL Item No. 91807 19.PJN174-14_GA-USA_HHD-Ohrthermometer_DSO364_28.07.14 Montag, 28. Juli 2014 14:37:38 USAD TABLE OF CONTENTS No. Topic Page 1.0 Definition of symbols 5 2.0 Application and functionality 6 2.1 Intended use 6 2.2 Field of application 7 3.0 Safety instructions 7 3.1 General safety instructions 7 3.3 Environment for which the DSO 364 device is not suited 9 3.4 Usage by children and adolescents 10 3.5 Information on the application of the device 10 4.0 Questions concerning body temperature 13 4.1 What is body temperature? 13 4.2 Advantages of measuring the body tempera- ture in the ear 14 4.3 Information on measuring the body tempera- ture in the ear 15 5.0 Scope of delivery / contents 16 6.0 Designation of device parts 17 7.0 LCD display 18 8.0 Basic functions 19 8.1 Commissioning of the device 19 8.2 Warning indicator if the body temperature is 21 too high 2 19.PJN174-14_GA-USA_HHD-Ohrthermometer_DSO364_28.07.14 Montag, 28. Juli 2014 14:37:38 TABLE OF CONTENTS USA No. Topic Page 8.3 Backlighting / torch function 21 8.4 Energy saving mode 22 8.5 Setting °Celsius / °Fahrenheit 22 9.0 Display / setting time and date 23 9.1 Display of time and date 23 9.2 Setting time and date 23 10.0 Memory mode 26 11.0 Measuring the temperature in the ear 28 12.0 Measuring the temperature on the forehead 30 13.0 Object temperature measurement 32 14.0 Disposal of the device 33 15.0 Battery change and information concerning batteries 33 16.0 Cleaning and care 36 17.0 “Cleaning” warning indicator 37 18.0 Calibration 38 19.0 Malfunctions 39 20.0 Technical specification 41 21.0 Warranty 44 3 19.PJN174-14_GA-USA_HHD-Ohrthermometer_DSO364_28.07.14 Montag, 28. -

NASA Eclips Educator Guide: Real World Mathematics
National Aeronautics and Space Administration NASA eClips™ Educator Guide Real World: Mathematics: Preparing for a Soft Landing Educational Product Educators & Students Grades 6-8 www.nasa.gov NP-2008-09-106-LaRC Preparing For A Soft Landing Preparing for a Soft Landing Grade Level: 6-8 Lesson Overview: Subjects: Students are introduced to the Orion Crew Exploration Middle School Mathematics Vehicle (CEV) and NASA’s plans to return to the Moon in this Physical Science lesson. Thinking and acting like engineers, they design and build models representing Orion, calculating the speed and acceleration of the models. Teacher Preparation Time: This lesson is developed using a 5E model of learning. This model is based upon 1 hour constructivism, a philosophical framework or theory of learning that helps students connect new knowledge to prior experience. In the ENGAGE section of the lesson, Lesson Duration: students learn about the Orion space capsule through the use of a NASA eClips video Five 55-minute class meetings segment. Teams of students design their own model of Orion to be used as a flight test model in the EXPLORE section. Students record the distance and time the models fall Time Management: and make suggestions to redesign and improve the models. Class time can be reduced to three 55-minute time blocks During the EXPLAIN section, students answer questions about speed, velocity and if some work is completed at acceleration after calculating the flight test model’s speed and acceleration. home. Working in teams, students redesign the flight test models to slow the models by National Standards increasing air resistance in the EXTEND section of this lesson. -

Pouring Plates from Prepared Bottled Media
Pouring Plates from Prepared Bottled Media Primary Hazard Warning Never purchase living specimens without having a disposition strategy in place. When pouring bottles, agar is HOT! Burning can occur. Always handle hot agar bottles with heat-protective gloves. For added protection wear latex or nitrile gloves when working with bacteria, and always wash hands before and after with hot water and soap. Availability Agar is available for purchase year round. Information • Storage: Bottled agar can be stored at room temperature for about six months unless otherwise specified. Never put agar in the freezer. It will cause the agar to breakdown and become unusable. To prevent contamination keep all bottles and Petri dishes sealed until ready to use. • Pouring Plates • Materials Needed: • Draft-free enclosure or Laminar flow hood • 70% isopropyl alcohol • Petri dishes • Microwave or hot water bath or autoclave 1. Melt the agar using one of the following methods: a) Autoclave: Loosen the cap on the agar bottle and autoclave the bottle at 15 psi for five minutes. While wearing heat-protective gloves, carefully remove the hot bottle and let it cool to between 75–55°C before pouring. This takes approximately 15 minutes. b)Water Bath: Loosen the cap on the agar bottle and place it into a water bath. Water temperature should remain at around 100°C. Leave it in the water bath until the agar is completely melted. While wearing heat- protective gloves, carefully remove the hot bottle and let it cool to between 75–55°C before pouring. c) Microwave: Loosen the cap on the agar bottle before microwaving. -

BROOKFIELD DIAL READING VISCOMETER with Electronic Drive
BROOKFIELD DIAL READING VISCOMETER with Electronic Drive Operating Instructions Manual No. M00-151-I0614 SPECIALISTS IN THE MEASUREMENT AND CONTROL OF VISCOSITY with offices in : Boston • Chicago • London • Stuttgart • Guangzhou BROOKFIELD ENGINEERING LABORATORIES, INC. 11 Commerce Boulevard, Middleboro, MA 02346 USA TEL 508-946-6200 or 800-628-8139 (USA excluding MA) FAX 508-946-6262 INTERNET http://www.brookfieldengineering.com TABLE OF CONTENTS I. INTRODUCTION .....................................................................................5 I.1 Components .......................................................................................................5 I.2 Utilities ................................................................................................................6 I.3 Specifications .....................................................................................................6 I.4 Set-Up ................................................................................................................7 I.5 IQ, OQ, PQ .........................................................................................................7 I.6 Safety Symbols and Precautions .......................................................................8 I.7 Cleaning .............................................................................................................8 II. GETTING STARTED ..............................................................................9 II.1 Operation ...........................................................................................................9 -

ELISA Plate Reader
applications guide to microplate systems applications guide to microplate systems GETTING THE MOST FROM YOUR MOLECULAR DEVICES MICROPLATE SYSTEMS SALES OFFICES United States Molecular Devices Corp. Tel. 800-635-5577 Fax 408-747-3601 United Kingdom Molecular Devices Ltd. Tel. +44-118-944-8000 Fax +44-118-944-8001 Germany Molecular Devices GMBH Tel. +49-89-9620-2340 Fax +49-89-9620-2345 Japan Nihon Molecular Devices Tel. +06-6399-8211 Fax +06-6399-8212 www.moleculardevices.com ©2002 Molecular Devices Corporation. Printed in U.S.A. #0120-1293A SpectraMax, SoftMax Pro, Vmax and Emax are registered trademarks and VersaMax, Lmax, CatchPoint and Stoplight Red are trademarks of Molecular Devices Corporation. All other trademarks are proprty of their respective companies. complete solutions for signal transduction assays AN EXAMPLE USING THE CATCHPOINT CYCLIC-AMP FLUORESCENT ASSAY KIT AND THE GEMINI XS MICROPLATE READER The Molecular Devices family of products typical applications for Molecular Devices microplate readers offers complete solutions for your signal transduction assays. Our integrated systems γ α β s include readers, washers, software and reagents. GDP αs AC absorbance fluorescence luminescence GTP PRINCIPLE OF CATCHPOINT CYCLIC-AMP ASSAY readers readers readers > Cell lysate is incubated with anti-cAMP assay type SpectraMax® SpectraMax® SpectraMax® VersaMax™ VMax® EMax® Gemini XS LMax™ ATP Plus384 190 340PC384 antibody and cAMP-HRP conjugate ELISA/IMMUNOASSAYS > nucleus Single addition step PROTEIN QUANTITATION cAMP > λEX 530 nm/λEM 590 nm, λCO 570 nm UV (280) Bradford, BCA, Lowry For more information on CatchPoint™ assay NanoOrange™, CBQCA kits, including the complete procedure for this NUCLEIC ACID QUANTITATION assay (MaxLine Application Note #46), visit UV (260) our web site at www.moleculardevices.com.