(Natrix Natrix) in Switzerland 2013 – 2015 1
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Ausgabe August/September 2018 Mitteilungsblatt Der Gemeinde Hüttlingen Herausgeber: Gemeindeverwaltung, Schule, Kirche Und Vereine THUR BLICK Gemeindemitteilungen
Ausgabe August/September 2018 Mitteilungsblatt der Gemeinde Hüttlingen Herausgeber: Gemeindeverwaltung, Schule, Kirche und Vereine THUR BLICK Gemeindemitteilungen Mitteilungen des Einwohneramtes Erneuerungswahlen Gemeinderat: Kandidatin oder Kandidat gesucht! Jubilare An der Berchtoldsgemeindeversammlung 2019 wer- 12. August Wüthrich, Ernst, Hüttlingen 86 den die Gesamterneuerungswahlen für den Gemein- 15. August Tanner, Bärbel, Eschikofen 80 derat stattfinden. 22. August Känzig, Elsy, Mettendorf 75 Bis auf Manfred Manser, Ressort Tiefbau und Um- 27. September Gessler, Hans Ulrich, Mettendorf 85 welt, stellen sich alle Bisherigen zur Wiederwahl. 03. September Langone, Raffaele, Eschikofen 80 Gesucht wird nun eine Kandidatin oder ein Kandidat 16. September Wegmüller, Rosa, Hüttlingen 75 für die Wahl in den Gemeinderat. Wir gratulieren den Jubilarinnen und Jubilaren ganz Sind Sie das neue Mitglied im Gemeinderat? herzlich. Wir wünschen Glück und Zufriedenheit, Gesucht wird ein neues Mitglied in den Gemeinderat vor allem aber gute Gesundheit. (Wahl an der Berchtoldsgemeinde 2019). Die wichtigste Voraussetzung ist das Interesse am Todesfälle Wohl unserer Gemeinde. Im Gegenzug bietet Ihnen 23. Mai Wirz, Martin, Hüttlingen das Amt eine interessante und verbindende Aufgabe, 9. Juni Wirz, Dora, Hüttlingen unterstützt von einem kollegialen Team. Wir sind gerne bereit, Sie in einem persönlichen Ge- Wir entbieten den Angehörigen unsere herzliche An- spräch über die zu erwartenden Aufgaben zu infor- teilnahme. mieren und von Ihnen zu erfahren, wo Ihre Interes- sen oder Stärken liegen. Publikation von Zivilstandmitteilungen Melden Sie sich auch, wenn Sie nicht selber kandi- Falls Sie nicht wünschen, dass Sie betreffende Mit- dieren wollen, aber eine ideale Person für ein solches teilungen im Thurblick erscheinen, teilen Sie uns das Amt zu kennen glauben. -

EFH Harrison Oberuzwil Bewertungsgutschten 2017.1
Kennzahlen Werte Ertragswert 920'000 CHF Ertrage und Kosten Mietwert (Soll-Ertrag) t otal Nutzungskosten total Betriebskosten Mietzinsrisiko Verwaltungskosten Unterhaltskosten Entwertung / lnstandsetzung Technische Entwertung Entwertungsanteil in % Ri.ickstellungen pro Jahr Flachen und Volumen Grundstocksflache GSF 2 762 m Gebaudevolumen GV 3 761 m Vermietbare Flache VMF 2 0 m Kennzahlen Verkehrswert / Gebaudevolu men GV 823 CHF/m3 Verkehrswert / Vermietbare Flache VMF 0 CHF/m2 Bruttorendite auf Mietertrag (1st) 4.38 % Mietwert / Vermi etbar e Flache VMF 0 CHF /m2 Nutzungskosten / Mietwert 25.41 % R0ckstellung / Mietwert 19 .14 % Kostenzuschlage Betriebskosten in % vom Ertragswert 0.18 % Mietzinsrisiko in % vom Ertragsw ert 0.00 % Verwaltungskosten in % vom Ertragswert 0.00 % Unterhaltskosten in % vom Ertragswert 0.58 % R0ckstellung in % vom Ertragswert 0.57 % EFH Harr ison , 9242 Ob er uzwil 2 Ausgangslage Besichtigung Die bestehende Bausubstanz wurde mittels einer einfachen Besichtigung beurteilt. Nicht zugangliche Bauteile wie unterputz verlegte Le itungen oder verkleidete Materialien wurden nicht freigelegt. Es wird angenommen , dass deren Zustand , insbesondere jegliche Warmedämmungen an Fassade und Dach dem normalen, zu erwartenden Zustand entsprechen. Bis auf den aufgestauten Erneuerungsbedarf wurden keine ausserordentlichen Verhältnisse festgestellt. Auf statische Berechnungen von tragenden Gebaudeteilen wurde verzichtet. Für versteckte Baumängel oder Bauschäden , die ohne Aufschlüsse nicht erkennbar sind , wird keine Haftung übernommen. Grundlagen I Unterlagen I Dokumente Grundbuchauszug Liegenschaft Nr. 1647 06 .02.2017 Schatzung der Steuerwerte Kanton St. Gallen inkl. 26 .09.2012 Berechnungsgrundlagen Gebaudeversicherungsnachweis 2014 23 .01 .2017 Grundriss - und Schnitt - und Fassadenplane undatiert Baueingabeplan Erstellung Wintergargen 23 .02 .1999 Besichtigung 17 .12.2016 Sachfragen I Rechtsfragen Verkehrswertgutachten haben in aller Regel nur Sachfragen zu beantworten . -

Graubünden for Mountain Enthusiasts
Graubünden for mountain enthusiasts The Alpine Summer Switzerland’s No. 1 holiday destination. Welcome, Allegra, Benvenuti to Graubünden © Andrea Badrutt “Lake Flix”, above Savognin 2 Welcome, Allegra, Benvenuti to Graubünden 1000 peaks, 150 valleys and 615 lakes. Graubünden is a place where anyone can enjoy a summer holiday in pure and undisturbed harmony – “padschiifik” is the Romansh word we Bündner locals use – it means “peaceful”. Hiking access is made easy with a free cable car. Long distance bikers can take advantage of luggage transport facilities. Language lovers can enjoy the beautiful Romansh heard in the announcements on the Rhaetian Railway. With a total of 7,106 square kilometres, Graubünden is the biggest alpine playground in the world. Welcome, Allegra, Benvenuti to Graubünden. CCNR· 261110 3 With hiking and walking for all grades Hikers near the SAC lodge Tuoi © Andrea Badrutt 4 With hiking and walking for all grades www.graubunden.com/hiking 5 Heidi and Peter in Maienfeld, © Gaudenz Danuser Bündner Herrschaft 6 Heidi’s home www.graubunden.com 7 Bikers nears Brigels 8 Exhilarating mountain bike trails www.graubunden.com/biking 9 Host to the whole world © peterdonatsch.ch Cattle in the Prättigau. 10 Host to the whole world More about tradition in Graubünden www.graubunden.com/tradition 11 Rhaetian Railway on the Bernina Pass © Andrea Badrutt 12 Nature showcase www.graubunden.com/train-travel 13 Recommended for all ages © Engadin Scuol Tourismus www.graubunden.com/family 14 Scuol – a typical village of the Engadin 15 Graubünden Tourism Alexanderstrasse 24 CH-7001 Chur Tel. +41 (0)81 254 24 24 [email protected] www.graubunden.com Gross Furgga Discover Graubünden by train and bus. -

Mehr Als Ein Altes Ritual Schulbehörde Kemmental
Nr. 24 Jahrgang 38 Freitag, 21. Dezember 2018 Erscheinungsweise: Freitag, 14-täglich LLeennggwwiilleerr ZZiiiittiigg Redaktionsschluss: Dienstag, 12 Uhr Amtliches Publikationsorgan der Gemeinde Lengwil Gemeinde Lengwil, Hauptstrasse 8, 8574 Lengwil Redaktion: Kreuzlinger Zeitung, Bahnhofstr. 33b, Postfach 1616, 8280 Kreuzlingen Tel. 071 686 30 00, www.lengwil.ch Tel. 071 678 80 34, E-Mail: [email protected] Mehr als ein altes Ritual Nach all dem vorweihnachtlichen ber, beginnt der Festgottesdienst erst Gottesdienst, w elcher von Pfr. Timo Kauf– und Festtrubel dieser Tage um 10.30 Uhr. Dieser findet in der Kir - Garthe und der Kreuzlinger Pastoral - bleibt für viele Menschen die Frage, che in Oberhofen statt und wird mit assistentin Christine Rammensee ge - wo denn nun die eigentliche Mitte Predigt, Liedern des Chörli und ei - staltet wird. Mit dabei sind einige des Weihnachtsfestes zu finden ist. nem gemeinsamen Abendmahl ge - Sternsinger, die während des Gottes - Genau deshalb bieten wir Ihnen auch feiert. dienstes ausgesandt werden. in diesem Jahr als Kirchgemeinde Am Stephanstag bietet sich um Lengwil eine reiche Auswahl an be - 9.45 Uhr in der Kirche Kurzricken - Besuch von den Sternensingern sinnlichen Gottesdiensten über die bach die Möglichkeit, an einem Re - Am Mittwoch, 9. Januar, werden sie Weihnachtstage an: gionalgottesdienst teilzunehmen. bei Menschen, die dies wünschen, Pfarrer Timo Garthe wird dort die vorbei gehen, ihre Lieder singen, ih - Unser Weihnachtsgottesdienst Predigt halten und Pfr. Damian Brot ren Segen fürs kommende Jahr wei - Am 24. Dezember um 17 Uhr wird für gestaltet die Liturgie. Wir freuen uns tergeben und für benachteiligte Kin - alle Familien mit jüngeren Kindern auf Sie. der sammeln. -

Abdankung/Bestattung Auf Dem Katholischen Friedhof)
Informationen für Angehörige bei einem Todesfall (Abdankung/Bestattung auf dem katholischen Friedhof) ______________________________________________________________________________________ Der Tod eines Angehörigen macht betroffen und löst Ängste aus, es entsteht Unsicherheit und es stellen sich viele Fragen. Wir alle gehen auf diesen Tag des Abschieds zu. Je eher wir uns dazu Gedanken ma- chen und unsere Anliegen den Angehörigen mittei- len, desto gelassener können wir miteinander über die letzten Dinge reden. Sich von einem geliebten Menschen endgültig ver- abschieden zu müssen, ist eine der schmerzlichsten Situationen, nehmen Sie sich genügend Zeit um Abschied zu nehmen. Im Zusammenhang mit einem Todesfall kommen ungewohnte Aufgaben und Behördengänge auf die Angehörigen zu. Das Bestattungsamt Romanshorn regelt für Sie beinahe alles, was mit der Überfüh- rung, der Abdankung und der Beisetzung zusam- menhängt. Sämtliche beteiligte Stellen werden durch das Bestattungsamt Romanshorn informiert. Dieses Merkblatt dient Ihnen als Übersicht über den Ablauf des Bestattungsgespräches sowie über weitere re- levante Vorkehrungen. Das Bestattungsamt derjenigen Gemeinde, wo die verstorbene Person ihren letzten gesetzlichen Wohnsitz hatte, ist zuständig für die anfallenden Aufgaben. Die involvierten Stellen (Einwohnerdiens- te / Bestattungsamt) geben in Bestattungsfragen gerne Auskunft. Mit diesen Informationen soll Ihnen der Umgang mit den anfallenden Formalitäten so leicht wie möglich gemacht werden. Es beinhaltet Informationen zum Ablauf und zu -

A New Challenge for Spatial Planning: Light Pollution in Switzerland
A New Challenge for Spatial Planning: Light Pollution in Switzerland Dr. Liliana Schönberger Contents Abstract .............................................................................................................................. 3 1 Introduction ............................................................................................................. 4 1.1 Light pollution ............................................................................................................. 4 1.1.1 The origins of artificial light ................................................................................ 4 1.1.2 Can light be “pollution”? ...................................................................................... 4 1.1.3 Impacts of light pollution on nature and human health .................................... 6 1.1.4 The efforts to minimize light pollution ............................................................... 7 1.2 Hypotheses .................................................................................................................. 8 2 Methods ................................................................................................................... 9 2.1 Literature review ......................................................................................................... 9 2.2 Spatial analyses ........................................................................................................ 10 3 Results ....................................................................................................................11 -
List & Label Report
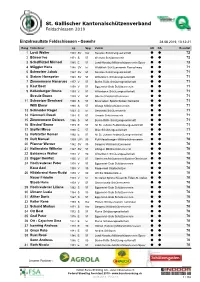
St. Gallischer Kantonalschützenverband Feldschiessen 2019 Einzelresultate Feldschiessen - Gewehr 28.08.2019, 10:12:21 Rang Teilnehmer Jg. Spg. Verein AK KA Resultat 1 Lusti Walter 1948 SV Kar Nesslau Schützengesellschaft 72 2 Büsser Ivo 1971 S 57 Weesen Schützenverein 72 3 Schafflützel Michael 1985 E 57 Laad-Nesslau Militärschützenverein Speer 72 4 Müggler Hans 1946 SV 57 Waldkirch Schützenverein Ramschwag 71 5 Schweizer Jakob 1947 SV 57 Nesslau Schützengesellschaft 71 6 Steiner Hanspeter 1948 SV 57 Wittenbach Schützengesellschaft 71 7 Zimmermann Hansruedi 1957 V 57 Buchs-Räfis Schützengesellschaft 71 8 Kast Beat 1958 V 57 Eggersriet-Grub Schützenverein 71 9 Kellenberger Bruno 1959 V 57 Wittenbach Schützengesellschaft 71 Streule Bruno 1959 V 57 Oberriet Feldschützenverein 71 11 Schweizer Bernhard 1960 S 57 Brunnadern Sportschützen Neckertal 71 Willi Elmar 1960 S 57 Wangs Militärschützenverein 71 13 Schneider Roger 1963 S 57 Sennwald Schützenverein 71 14 Hämmerli Ruedi 1964 S 57 Uznach Schützenverein 71 15 Zimmermann Dolores 1966 S 57 Buchs-Räfis Schützengesellschaft 71 16 Bischof Bruno 1979 E 57 Alt St. Johann Feldschützengesellschaft 71 17 Stoffel Mirco 1990 E 57 Mols Schützengesellschaft 71 18 Hofstetter Roman 1992 E 57 Alt St. Johann Feldschützengesellschaft 71 19 Duft Manuel 1999 U21 90 Rufi-Maseltrangen Militärschützenverein 70 20 Pfanner Werner 1942 SV 90 Sargans Militärschützenverein 70 21 Hollenstein Wilhelm 1947 SV 57 Libingen Militärschützenverein 70 22 Baldamus Walter 1953 V 90 Wittenbach Schützengesellschaft 70 23 Bigger Bonifaz 1955 V 57 Oberterzen Schützenverein Quarten-Oberterzen 70 24 Hochreutener Peter 1958 V 57 Eggersriet-Grub Schützenverein 70 Kauz Axel 1958 V 90 Rapperswil Stadtschützen 70 26 Hildebrand Hans-Rudolf 1959 V 90 Wil SG Stadtschützen 70 Nauer Fridolin 1959 V 57 St. -

2008 FSG Berschis
25. St.Georgenschiessen 2008 FSG Berschis Oberterzen, Schützenverein Quarten-Oberterzen Bless Roger Schilsstrasse 15 8890 Flums Vereinsabrechnung Vereinswettkampf Vereinskategorie 2 Vereinsschützen / U20 / Pflichtteilnehmer 23/ 2 / 10 Pflichtteilnehmer Gubser Heiny V 90 77 Bless Roger E Std 77 Pfiffner Martin S Std 76 Janser Bettina E Std 76 Gubser Adrian E Std 75 Schnyder Peter V 90 74 Bigger Patrick J 90 73 Bless Othmar V Std 73 Giger Othmar S 57 03 73 Bigger Ursula S 90 73 747 Nicht-Pflichtteilnehmer Zeller Werner S 57 03 73 Bigger Peter E Std 73 Zeller Roman E Std 73 Bigger Peter S 90 72 Gubser Emil S 90 72 Gubser Simon E Std 71 Rupf Susy S 57 03 69 Gubser Ueli V 90 69 Gubser Adrian J Std 69 Kessler Isidor S 90 68 Bigger Bonifaz S Std 68 Gubser Hermann E 90 64 Kessler Ida V 90 62 903 VereinsWK 3.0 copyright by Indoor Swiss Shooting AG, 9200 Gossau www.IndoorSwiss.ch 25. St.Georgenschiessen 2008 FSG Berschis Pflichtresultat + Zuschlag Nichtpflichtresultate / Pflichtteilnehmer = Resultat 747 18.060 10 76.506 VereinsWK 3.0 copyright by Indoor Swiss Shooting AG, 9200 Gossau www.IndoorSwiss.ch 25. St.Georgenschiessen 2008 FSG Berschis Einzelresultate Sportgerät Resultat Auszeichnung Auszahlung 156462, Bigger Bonifaz, Quarten, 1955, S Vereinsstich Std 68 284643, Bigger Patrick, Quarten, 1990, J Vereinsstich 90 73 Kranzkarte 156459, Bigger Peter, Quarten, 1959, S Vereinsstich 90 72 Kranzkarte 156460, Bigger Peter, Quarten, 1986, E Vereinsstich Std 73 Kranzkarte 156461, Bigger Ursula, Quarten, 1960, S Vereinsstich 90 73 Kranzkarte 113088, -

Stability of Travel Behaviour: Thurgau 2003
Research Collection Working Paper Stability of Travel Behaviour: Thurgau 2003 Author(s): Löchl, Michael Publication Date: 2005 Permanent Link: https://doi.org/10.3929/ethz-b-000066687 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Stability of Travel Behaviour: Thurgau 2003 Michael Löchl Travel Survey Metadata Series 16 Travel Survey Metadata Series 16 Stability of Travel Behaviour: Thurgau 2003 Michael Löchl IVT ETH Zürich Zürich Phone: +41 44 633 62 58 Fax: +41 44 633 10 57 [email protected] Abstract Within the project, a six week travel survey has been conducted among 230 persons from 99 households in Frauenfeld and the surrounding areas in Canton Thurgau from August until December 2003. The design built on the questionnaire used in the German project Mobidrive, but developed the set of questions further. All trip destinations of the survey have been geocoded. Moreover, route alternatives for private motorised transport and public transport have been calculated. Moreover, the collected data has been compared with the National Travel Survey 2000 (Mikrozensus zum Verkehrsverhalten 2000), whereas differences in terms of sociodemographic characteristics of the respondents and particularly their travel behaviour couldn't be observed except for an higher proportion of GA and Halbtax ownership. For example, the average trip frequency per person and day is almost the same. In order to check for possible fatigue effects of the amount of reported trips, several GLM (Generalised Linear Model) and poisson regression models have been estimated besides descriptive analysis. -

Mitteilungsblatt Der Gemeinde Oberuzwil Gemeinde Oberuzwil
Ausgabe 05/2016 · 11. März 2016 Mitteilungsblatt der Gemeinde Oberuzwil Gemeinderat, Verwaltung Zwei Kantonsräte aus Oberuzwil Osterhasen und noch viel mehr Revision der Gemeindeordnung Schulen Besuchstage Primarschulen Lehrlingsabend an der Oberstufe Aktuelles aus der Musikschule Vereine, Institutionen Einladung zur Vorgemeinde Hauptversammlung FG Bichwil Raum für Kunst und Kultur Veranstaltungskalender Gemeinde Oberuzwil Wahlen/Abstimmungen Wohnheim Bisacht Zwei Kantonsräte aus Osterhasen und noch Oberuzwil viel mehr Die Kantonsratswahlen vom 28. Februar 2016 sind für In der Kreativwerkstatt des Wohnheims Bisacht ist die Gemeinde Oberuzwil erfolgreich und erfreulich zu- auch dieses Jahr ein vielseitiges Angebot an Oster- und gleich verlaufen. Der bisherige Kantonsrat Ernst Dobler Frühlingsdekorationen vorbereitet worden, das am (CVP) wurde glanzvoll wiedergewählt und neu nimmt «Oster-Kafi» auf glückliche Kundschaft wartet. auch Cornel Egger (CVP) mit einem sehr guten Wahlre- sultat Einsitz im Kantonsparlament – dies als einziger Gemeindepräsident im Wahlkreis Wil. Der Gemeinderat gratuliert den Gewählten herzlich zur ehren- vollen (Wieder-)Wahl und freut sich, dass ab 1. Juni 2016 wie- der zwei Oberuzwiler die Kantonspolitik mitbestimmen und sich in St.Gallen für die Gemeinde Oberuzwil und für die Region einsetzen werden. Ebenfalls gute Wahlresultate Nebst den Gewählten haben erfreulicherweise weitere Ein- wohnerinnen und Einwohner der Gemeinde Oberuzwil für einen Sitz im Kantonsrat kandidiert und respektable Wahl- resultate erzielt. -

Mols (Quarten) 5984
Kt. Bez. Gemeinde Ort ISOS 1 SG 09 Quarten Mols 1. Fassung 10.1999/fsr Bearbeitungsprotokoll def. 03.07.2002/fsr Siedlungsentwicklung Geschichte und historisches Wachstum In der Nähe des am östlichen Walensee liegenden Orts wurden römische Münzen gefun- den. Ob jedoch der für das Mittelalter nachweisbare Landweg auf der Südseite des Walensees schon in der Römerzeit existierte, ist fraglich. 1178 wurde Mols in einer päpstlichen Urkunde als Besitz des Klosters Schänis erwähnt. Aus dem Jahre 1470 ist ein Alpbrief überliefert. 1475 gab es für Mols die erste Weideordnung. Ende des 14. Jahrhunderts gehörte der Ort zur Herrschaft Windegg (Gaster). Noch im 15. Jahr- hundert wurden Mols, Terzen und Walenstadt von der Landvogtei Gaster getrennt und mit der Landvogtei Sargans vereinigt. Kirchlicher Mittelpunkt war Walenstadt. Der Bau der ersten Kapelle in Mols geht auf das Jahr 1725 zurück. 1787 löste sich Mols von der Kirche Walenstadt und bildete eine eigene Pfarrei. Die heutige, den Ortskern akzentuierende Pfarrkirche (1.0.1) entstand in den Jahren 1862-65. Ältere Quellen erwähnen 1821 als Jahr der Einweihung und 1822 als Baujahr des Kirchturms. Mit Walenstadt war Mols Jahrhunderte lang als Ausburgergemeinde verbunden und stell- te bis 1803 einen Stadtrat. Seit der Neugründung des Kantons St. Gallen ist Mols Teil der Gemeinde Quarten. Die Siegfriedkarte von 1897 zeigt, wie die 1859 eröffnete Eisenbahnlinie Ziegel- brücke–Sargans (0.0.10) einen schmalen Uferstreifen vom Dorf abtrennt. Hangfuss und unterer Hangabschnitt sind dispers besiedelt: kleine Verdichtungen sind auf der Karte bei der Kirche, bei der Säge und Mühle und bei Massraga auszumachen. Haupt- verkehrsachse war schon damals die ab 1848 durchgehend befahrbare Landstrasse Kerenzerberg–Walenstadt. -

Fladeblatt.Html/16 Oder
PDF AUF DER HOMEPAGE DER GEMEINDEN FlADE BLATT INFORMATIONSBLATT DER GEMEINDEN FLAWIL UND DEGERSHEIM 6. JAHRGANG | AUSGABE 10 | 5. MÄRZ 2021 PLANAUFLAGE Den Kontakt zu den Jugendlichen Im Zuge des Wasserbauprojekts Bueben- taler- und Aeschbach wird die Brücke beim nicht verlieren Bubentalweg ersetzt. Das Bauvorhaben sieht eine einfache Brücke für Fussgängerinnen und Fussgänger sowie für Radfahrerinnen und Radfahrer vor. ››› SEITE 2 BIOABFALL Die Trennung von Bioabfällen vom nor- malen Abfall spart nicht nur Geld, sondern trägt auch viel zur Schonung der Umwelt bei. So konnten im letzten Jahr in Degersheim 45 Tonnen CO2 eingespart werden. ››› SEITE 9 Das OJA-Team mit einheitlichem Look (von links): Domenica Del Tiglio, René Hirschi und Stellenleiter Tobias Marti. FLAWIL Seit bald einem Jahr wird das Leben Monate zurückblickt. Vor allem im März und durch das Coronavirus bestimmt. Auch für die April 2020, als der Jugendtreff geschlossen blei- Mitarbeitenden der Offenen Jugendarbeit ben musste, war das OJA-Team ganz besonders (OJA) waren die vergangenen zwölf Monate gefordert. «Die Beziehung ist das Kernelement FAHRVERBOT eine Herausforderung. Immer wieder mussten der Offenen Jugendarbeit. Ein professioneller Die Gemeinden Degersheim und Neckertal sie die Angebote anpassen. «In erster Linie Beziehungsaufbau ist aber nur schwer möglich, haben beschlossen, das Fahrverbot auf der ging es darum, den Kontakt zu den Jugendli- wenn der Jugendtreff geschlossen ist», sagt Tobias Berg-Wolfensbergstrasse auf alle Wochen- chen nicht zu verlieren», sagt Tobias Marti, Marti. Die OJA-Mitarbeitenden mussten nach tage auszudehnen. Das Verbot gilt ab sofort. Stellenleiter der Offenen Jugendarbeit Flawil. neuen Wegen suchen und fanden sie vor allem in digitalen Angeboten. Diese hatten in erster Linie ››› SEITE 9 Ein Jahr dauert die Corona-Krise nun schon an.