Tricare Prenatal Chewable Tablet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
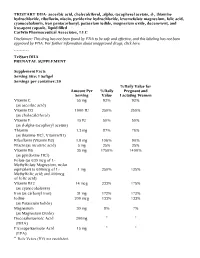
TRISTART DHA- Ascorbic Acid, Cholecalciferol, .Alpha
TRISTART DHA- ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, d-, thiamine hydrochloride, riboflavin, niacin, pyridoxine hydrochloride, levomefolate magnesium, folic acid, cyanocobalamin, iron pentacarbonyl, potassium iodide, magnesium oxide, doconexent, and icosapent capsule, liquid filled CarWin Pharmaceutical Associates, LLC Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here. ---------- TriStart DHA PRENATAL SUPPLEMENT Supplement Facts Serving Size: 1 Softgel Servings per container: 30 %Daily Value for Amount Per %Daily Pregnant and Serving Value Lactating Women Vitamin C 55 mg 92% 92% (as ascorbic acid) Vitamin D3 1000 IU 250% 250% (as cholecalciferol) Vitamin E 15 IU 50% 50% (as d-alpha-tocopheryl acetate) Thiamin 1.3 mg 87% 76% (as thiamine HCl, Vitamin B1) Riboflavin (Vitamin B2) 1.8 mg 106% 90% Niacin (as nicotinic acid) 5 mg 25% 25% Vitamin B6 35 mg 1750% 1400% (as pyridoxine HCl) Folate (as 630 mcg of L- Methylfolate Magnesium, molar equivalent to 600mcg of L- 1 mg 250% 125% Methylfolic acid; and 400mcg of folic acid) Vitamin B12 14 mcg 233% 175% (as cyanocobalamin) Iron (as carbonyl iron) 31 mg 172% 172% Iodine 200 mcg 133% 133% (as Potassium Iodide) Magnesium 30 mg 8% 7% (as Magnesium Oxide) Docosahexaenoic Acid 200mg * * (DHA) Eicosapentaenoate Acid 15 mg * * (EPA) * Daily Values (DV) not established. OTHER INGREDIENTS: Gelatin (bovine), Glycerin, Purified Water, Yellow Bees Wax, Caramel Powder, Soy Lecithin, Natural Orange Flavor, Ethyl Vanillin. Contains: Soy and Fish TriStart DHA™ Softgel capsules are dye free, lactose, gluten and sugar free. -

Enbrace® HR DESCRIPTION: INGREDIENTS
EnBrace® HR with DeltaFolate ™ [1 NF Units] [15 mg DFE Folate] ANTI-ANEMIA PREPARATION as extrinsic/intrinsic factor concentrate plus folate. Prescription Prenatal/Vitamin Drug For Therapeutic Use Multi-phasic Capsules (30ct bottle) NDC 64661-650-30 Rx Only [DRUG] GLUTEN-FREE DESCRIPTION: EnBrace® HR is an orally administered prescription prenatal/vitamin drug for therapeutic use formulated for female macrocytic anemia patients that are in need of treatment, and are under specific direction and monitoring of vitamin B12 and vitamin B9 status by a physician. EnBrace® HR is intended for women of childbearing age who are – or desire to become, pregnant regardless of lactation status. EnBrace® HR may be prescribed for women at risk of depression as a result of folate or cobalamin deficiency - including folate-induced postpartum depression, or are at risk of folate-induced birth defects such as may be found with spina bifida and other neural tube defects (NTDs). INGREDIENTS: Cobalamin intrinsic factor complex 1 NF Units* * National Formulary Units (“NF UNITS”) equivalent to 50 mcg of active coenzyme cobalamin (as cobamamide concentrate with intrinsic factor) ALSO CONTAINS: 1 Folinic acid (B9-vitamer) 2.5 mg + 1 Control-release, citrated folic acid, DHF (B9-Provitamin) 1 mg 2 Levomefolic acid (B9 & B12- cofactor) 5.23 mg 1 6 mg DFE folate (vitamin B9) 2 9 mg DFE l-methylfolate magnesium (molar equivalent). FUNCTIONAL EXCIPIENTS: 13.6 mg FeGC as ferrous glycine cysteinate (1.5 mg 3 3,4 elemental iron ) [colorant], 25 mg ascorbates (24 mg magnesium l-ascorbate, 1 mg zinc l-ascorbate) [antioxidant], at least 23.33 mg phospholipid-omega3 complex5 [marine lipids], 500 mcg betaine (trimethylglycine) [acidifier], 1 mg magnesium l-threonate [stabilizer]. -

Levomefolic Acid | Medchemexpress
Inhibitors Product Data Sheet Levomefolic acid • Agonists Cat. No.: HY-14781 CAS No.: 31690-09-2 Molecular Formula: C₂₀H₂₅N₇O₆ • Molecular Weight: 459.46 Screening Libraries Target: Endogenous Metabolite; Reactive Oxygen Species Pathway: Metabolic Enzyme/Protease; Immunology/Inflammation; NF-κB Storage: Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month SOLVENT & SOLUBILITY In Vitro H2O : 5 mg/mL (10.88 mM; Need ultrasonic) DMSO : < 1 mg/mL (insoluble or slightly soluble) Mass Solvent 1 mg 5 mg 10 mg Concentration Preparing 1 mM 2.1765 mL 10.8823 mL 21.7647 mL Stock Solutions 5 mM 0.4353 mL 2.1765 mL 4.3529 mL 10 mM 0.2176 mL 1.0882 mL 2.1765 mL Please refer to the solubility information to select the appropriate solvent. BIOLOGICAL ACTIVITY Description Levomefolic acid (5-MTHF) is the natural, active form of folic acid used at the cellular level for DNA reproduction, the cysteine cycle and the regulation of homocysteine among other functions. IC50 value: Target: Folate analogLevomefolic acid has been proposed for treatment of cardiovascular disease and advanced cancers such as breast and colorectal cancers. Levomefolic acid (5-MTHF) has the prominent antioxidant activity. A high dose of 5-MTHF or folic acid does not influence Natural killer (NK) cell function in vitro. CUSTOMER VALIDATION • J Mol Med (Berl). 2019 Aug;97(8):1183-1193. See more customer validations on www.MedChemExpress.com Page 1 of 2 www.MedChemExpress.com REFERENCES [1]. Hirsch S, et al. Natural killer cell cytotoxicity is not regulated by folic acid in vitro. -

DENOVO- Levomefolic Acid Capsule Magna Pharmaceuticals, Inc
DENOVO- levomefolic acid capsule Magna Pharmaceuticals, Inc. ---------- Denovo Supplement Facts Serving Size: 1 capsule Servings per Container: 30 Each capsule contains the following: Folate [as 15,000mcg (as 15mg) DFE (dietary folate equivalents) L-5 MTHF (from L-5 methyltetrahydrofolate calcium salt)] Other Ingredients: Microcrystalline cellulose, capsule (gelatin,titanium dioxide, FD&C yellow #5, FD&C yellow #6), vegetable magnesium stearate, and silica Warning and Precautions Folic Acid, when administered in daily doses above 0.1mg may obscure the detection of B12 deficiency (specifically, the administration of folic acid may reverse the hematological manifestations of B12 deficiency, including pernicious anemia, while not addressing the neurological manifestations). 5- MTHF may be less likely than foli acid to mask vitamin B12 deficiency. Folate therapy alone is inadequate for the treatment of a B12 deficiency. A major depressive episode may be the inital presentation of bipolar disorder. It is generally believed, although not established in controlled trials, that treating such an episode with an antidepressant alone may increase the likelihood of a precipitation of mixed/manic episode in patients at risk for bipolar disorder. Denovo is not an antidepressant. However, 5-MTHF has been shown to enhance antidepressant effects of known antidepressants. Caution is recommended in patients with a history of bipolar illness. Patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder since mood elevation in this population is possible. Denovo should always be used under medical supervision. Safe Handling Before using this product, tell your doctor or pharmacist of all of the products you use. -

B-Supreme™ Comprehensive B Vitamin Formula Featuring Quatrefolic® and Coenzyme Q10
B-Supreme™ Comprehensive B vitamin formula featuring Quatrefolic® and Coenzyme Q10 B-SupremeTM benefits: OVERVIEW • Helps to reduce symptoms of stress This powerful and effective B vitamin combination formula supplies and enhance energy levels B vitamins in their preferred coenzymated forms, where possible, • Helps to maintain and support energy so the body does not have to phosphorylate them in order to be production in cells used in biochemical reactions. B-Supreme contains our proprietary • Helps to convert food into energy Quatrefolic® blend of active isomer, naturally-occurring folates. • Contains the most bioavailable active Choline and DMG are included to help support efficient forms of B vitamins methylation for optimal genetic expression. KEY FEATURES: History of B Vitamins B vitamins are a family of water-soluble nutrients that were discovered together at the beginning of the 20th century, initially thought simply to be “vitamin B”. As nutrition advanced, however, it was learned that they are in fact a family of compounds, each with a distinct role to play in promoting health. B vitamins are now known to play critical roles in modulating biochemistry and metabolism and represent the most common vitamins utilised by the body as cofactors for facilitating enzymatic function. The modern refined diet, high in sugar, alcohol, and devitalised foods, leads to lower levels of B vitamins. Many medications and stress may lower B vitamin levels. While B vitamins have mainly been researched individually, they also have therapeutic power when taken together. For example, a B complex supplement can relieve nocturnal leg cramps in the elderly.1 Thiamin (Vitamin B1): Nerve, Muscle and Brain Health Vitamin B1 is needed for energy production, heart function, and the health of the brain and nervous system. -

An in Silico Drug Repurposing for COVID-19[Version 1; Peer Review: 2
F1000Research 2020, 9:1166 Last updated: 22 JUL 2021 RESEARCH ARTICLE Targeting SARS-CoV-2 RNA-dependent RNA polymerase: An in silico drug repurposing for COVID-19 [version 1; peer review: 2 approved] Krishnaprasad Baby1*, Swastika Maity1*, Chetan H. Mehta2, Akhil Suresh2, Usha Y. Nayak2, Yogendra Nayak 1 1Department of Pharmacology, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal, Karnataka, 576104, India 2Department of Pharmaceutics, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal, Karnataka, 576104, India * Equal contributors v1 First published: 23 Sep 2020, 9:1166 Open Peer Review https://doi.org/10.12688/f1000research.26359.1 Latest published: 23 Sep 2020, 9:1166 https://doi.org/10.12688/f1000research.26359.1 Reviewer Status Invited Reviewers Abstract Background: The coronavirus disease 2019 (COVID-19) pandemic, 1 2 caused by severe acute respiratory syndrome coronavirus-2 (SARS- CoV-2), took more lives than combined epidemics of SARS, MERS, version 1 H1N1, and Ebola. Currently, the prevention and control of spread are 23 Sep 2020 report report the goals in COVID-19 management as there are no specific drugs to cure or vaccines available for prevention. Hence, the drug 1. Harish Holla, Central University of repurposing was explored by many research groups, and many target proteins have been examined. The major protease (Mpro), and RNA- Karnataka, Kalaburagi, India dependent RNA polymerase (RdRp) are two target proteins in SARS- 2. Hemachandra Reddy , Texas Tech CoV-2 that have been validated and extensively studied for drug development in COVID-19. The RdRp shares a high degree of University Health Sciences Center, Lubbock, homology between those of two previously known coronaviruses, USA SARS-CoV and MERS-CoV. -

L-METHYL-B6-B12 Tablets
L-METHYL-B6-B12 - levomefolate calcium, pyridoxal phosphate anhydrous, and methylcobalamin tablet, coated AvKARE, Inc. ---------- L-METHYL-B6-B12 Tablets DESCRIPTION L-Methyl-B6-B12 is a medical food for the clinical dietary management of the metabolic imbalances associated with hyperhomocysteinemia that cannot be managed by diet modification alone. Dispense by prescription. Use under medical supervision. Each round coated purple colored tablet contains: Pyridoxal 5'-phosphate 35 mg L-methylfolate Calcium 3 mg Methylcobalamin 2 mg Dietary Ingredients Dibasic Calcium Phosphate Dihydrate, Microcrystalline Cellulose 90, PYRIDOXAL-5'-PHOSPHATE, Microcrystalline Cellulose HD 90, Opadry II Purple 40L10045 (Polydextrose, Titanium Dioxide, Hypromellose 3cP, Hypromellose 6cP, Glycerol Triacetate, Hypromellose 50cP, FD&C Blue #2, FD&C Red #40 and Polyglycol 8000), Microcrystalline Cellulose 50, Opadry II Clear Y-19-7483 (Hypromellose 6cP, Maltodextrin, Hypromellose 3cP, Polyglycol 400 and Hypromellose 50cP), L- METHYLFOLATE CALCIUM, Magnesium Stearate, METHYLCOBALAMIN, and Carnauba Wax. Contains FD&C Blue #2 and FD&C Red #40. Indications Hyperhomocysteinemia of any etiology. Intended Use Medical foods are intended for the patient who has a limited or impaired capacity to ingest, digest, absorb, or metabolize ordinary foodstuffs or certain nutrients, or who has other special medically determined nutrient requirements, the dietary management of which cannot be achieved by the modification of the normal diet alone1. L-Methyl-B6-B12 is a specially formulated medical food for the dietary support of patients with hyperhomocysteinemia. It also is helpful in managing patients with high total homocysteine concentrations associated with malabsorption of vitamin B12 or suboptimal intake of B vitamins. L-Methyl-B6-B12 Tablets should always be used under medical supervision. -

Plasma Methylation Profile RESOURCE GUIDE
Plasma Methylation Profile RESOURCE GUIDE Science + Insight doctorsdata.com Methionine SAMe DNA SHMT THF RNA 5, 10 Methyltransferases MethyleneTHF Protein Thymidine DMG Lipids synthesis B12 BHMT SAH dUMP MTRR MTR TMG adenosine AHCY MTHFR Homocysteine 5 Methyl THF CBS Cystathionine Cysteine Sulte SUOX Sulfate Introduction The Plasma Methylation Profile is a functional assessment of the enzymes involved in methionine metabolism and the trans-sulfuration pathway (commonly called the “Methylation Pathway”). The genomics revolution has made it possible to assess genetic information stored in the DNA code. An awareness of single nucleotide polymorphisms (SNPs) has made genetic testing for certain SNPs part of diagnostic patient assessment. While the identification of SNPs in a patient’s genome is important, it is vital to remember that functional testing of enzymes should determine treatment decisions. There are many layers of translation between the genome and the enzyme. Enzyme function may be compromised not only by inheritance, but also by acquired epigenetic factors such as nutritional status, oxidative stress, autoimmunity or environmental exposures. There is mounting evidence that, especially within the folate and methylation pathways, multiple SNPs in multiple genes (haplotypes) may be necessary to alter metabolism or change health outcomes. Gastrointestinal functions may influence absorption, physiology, metabolism and immunity; nutrient maldigestion or malabsorption may inhibit normal enzyme functions, and may have greater effects on enzymes with SNPs. © 2016 Doctor’s Data, Inc. All rights reserved. doctorsdata.com Doctor’s Data, Inc. Plasma Methylation Enzyme and Nutrition Guide 2 Methionine High Methionine may be elevated for a variety of reasons. Several enzymes involved in the metabolism of methionine require magnesium and other nutritional cofactors. -

The Changing Faces of Vitamin B12 and Folic Acid – Part Two: Integration of Symptoms, and Genetic Testing to Select the Best Drugs for Your Patient
The Changing Faces of Vitamin B12 and Folic Acid – Part two: Integration of symptoms, and genetic testing to select the best drugs for your patient By: Dan Carter, ND The symptoms of vitamin B12 deficiency range from mild to severe, and in many cases are not specific enough to confirm a diagnosis. Symptoms can include diarrhea or constipation, fatigue, light headedness on standing, poor appetite, pallor, difficulty concentrating, shortness of breath on exertion, glossitis. Neurologic symptoms can appear after chronic deficiency and involve changes in mental status, depression, peripheral neuropathy, loss of balance. [Anemia – B12 deficiency. http://www.nlm.nih.gov/medlineplus/ency/article/000574.htm] Some of the symptoms of folate deficiency overlap with B12, including fatigue and glossitis, but others such as accelerated graying of hair, oral ulcers, and poor growth rates do not. [Folate deficiency. http://www.nlm.nih.gov/medlineplus/ency/article/000354.htm] The indicated blood tests for B12 deficiency are complete blood count (CBC), reticulocyte count, serum LDH and vitamin B12, while those for folate deficiency are CBC, serum and RBC folate. Other useful tests are serum homocysteine and methylmalonic acid (MMA). Serum homocysteine will be high with both deficiencies, while only MMA will be increased in B12 deficiency. [Vitamin B12 and Folate. http://labtestsonline.org/understanding/analytes/vitamin-b12/tab/test] As genetic testing has become more widely available, specific analysis of known single nucleotide polymorphisms (SNP) is increasingly accepted as an aid to diagnosis. In the case of folate metabolism several SNP have been identified as significant; the first SNP to gain widespread attention affected the MTHFR gene. -

Download CMI (PDF)
Quatrefolic® Primary biologically active form of folate | Levomefolic acid Consumer Medicine Information 1. What is in the leaflet? 1. You have any allergies to any other 5. While you are taking Quatrefolic® medicines, foods, preservatives, dyes This leaflet answers some of the more or additives. Things you MUST do: common questions about Quatrefolic® If you experience any allergic reaction 1. If you become pregnant while taking capsules. or other side effects after consuming Quatrefolic®, tell your healthcare It does not contain all the available Quatrefolic® you should discontinue use professional immediately. information. It does not take the place of and consult with your healthcare 2. If you are about to start taking a new talking to your healthcare professional. professional. medicine, tell your healthcare All medicines have risks and benefits. Your 2. You are pregnant or planning to professional that you are taking doctor has weighed the risks of you taking become pregnant. Quatrefolic®. Quatrefolic® capsules against the benefits 3. You are breastfeeding or planning to 3. If you are planning to have surgery, they expect it will have for you. breastfeed. including dental surgery, tell your If you have any concerns about taking this 4. You have or have had any other healthcare professional that you are medicine, ask your healthcare professional. health problems or issues including: taking Quatrefolic®. Keep this leaflet with the medicine. Iron deficiency (anemia) or think that 4. Always follow your healthcare You may need to read it again. you might be anemic. professional’s instructions carefully. 5. You drink large amounts of alcohol. Things you MUST NOT do: 2. -

Enbrace HR™ Multiphasic Soft Gelatin Capsules
ENBRACE HR- levomefolate magnesium, leucovorin, folic acid, ferrous cysteine glycinate, magnesium ascorbate, zinc ascorbate, cocarboxylase, flavin adenine dinucleotide, nadh, pyridoxal phosphate anhydrous, cobamamide, betaine, magnesium l-threonate, 1,2- docosahexanoyl-sn-glycero-3-phosphoserine calcium, 1,2-icosapentoyl-sn-glycero-3- phosphoserine calcium, and phosphatidyl serine capsule, delayed release pellets JAYMAC Pharmaceuticals, LLC Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here. ---------- EnBrace HR™ Multiphasic Soft Gelatin Capsules EnBrace® HR with DeltaFolate™ [15 mg DFE Folate ][50 mcg CBl] [1.5 mg FeGC] ANTI-ANEMIA PREPARATIONas folate, cobalamin & low-iron Prescription Prenatal Vitamin DrugFor Therapeutic Use Multi-phasic Capsules (30ct bottle) NDC 64661-650-30 R x Only [DRUG] GLUTEN-FREE DESCRIPTION: EnBrace® HR is an orally administered prescription prenatal vitamin drug for therapeutic use formulated to meet the unique nutritional needs of pregnancy - regardless of lactation status. EnBrace® HR may be taken by female macrocytic anemia patients that are in need of treatment and are under specific direction and monitoring of vitamin B9 and vitamin B12 status by a physician. EnBrace® HR is intended for women of childbearing age, and may be prescribed for women at risk of folate or cobalamin deficiency - including folate-induced perinatal and postpartum depression, and are at risk of folate-induced birth defects such as may be found with spina bifida and other neural tube defects (NTDs). EnBrace® HR is intended to address the increased need for metabolically-active variants of vitamin B9-vitamers in the cerebrospinal fluid, plasma, and/or red blood cells - as may be found in the SNP (Single-Nucleotide Polymorphisms) known as MTHFR (MethyleneTetraHydroFolate Reductase). -

L-METHYL-B6-B12 Tablets
L-METHYL-B6-B12- levomefolate calcium, pyridoxal phosphate anhydrous, and methylcobalamin tablet, coated Virtus Pharmaceuticals ---------- L-METHYL-B6-B12 Tablets L-Methyl-B6-B12 Tablets is an orally administered medical food for the dietary management of endothelial dysfunction in patients with diabetic peripheral neuropathy. Description Each round coated purple colored tablet contains: L-methylfolate Calcium 3 mg Pyridoxal 5'-phosphate 35 mg Methylcobalamin 2 mg Ingredients Dibasic Calcium Phosphate Dihydrate, Microcrystalline Cellulose 90, Pyridoxal-5'-Phosphate, Microcrystalline Cellulose HD 90, Opadry II Purple 40L10045 (Polydextrose, Titanium Dioxide, Hypromellose 3cP, Hypromellose 6cP, Glycerol Triacetate, Hypromellose 50cP, FD&C Blue #2, FD&C Red #40 and Polyglycol 8000), Microcrystalline Cellulose 50, Opadry II Clear Y-19-7483 (Hypromellose 6cP, Maltodextrin, Hypromellose 3cP, Polyglycol 400 and Hypromellose 50cP), Lmethylfolate Calcium, Magnesium Stearate, Methylcobalamin, and Carnauba Wax. L-Methyl-B6-B12 Tablets do not contain sugar, lactose, yeast or gluten. Pharmacology L-methylfolate or 6(S)-5-methyltetrahydrofolate [6(S)-5-MTHF], is the primary biologically active diastereoisomer of folate1 and the primary form of folate in circulation.2 It is also the form which is transported across membranes into peripheral tissues3, particularly across the blood brain barrier.4 In the cell, 6(S)-5-MTHF is used in the methylation of homocysteine to form methionine and tetrahydrofolate (THF).1 THF is the immediate acceptor of one carbon