Enbrace® HR DESCRIPTION: INGREDIENTS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications
International Journal of Molecular Sciences Review Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications Daniel Fernández-Villa 1, Maria Rosa Aguilar 1,2 and Luis Rojo 1,2,* 1 Instituto de Ciencia y Tecnología de Polímeros, Consejo Superior de Investigaciones Científicas, CSIC, 28006 Madrid, Spain; [email protected] (D.F.-V.); [email protected] (M.R.A.) 2 Consorcio Centro de Investigación Biomédica en Red de Bioingeniería, Biomateriales y Nanomedicina, 28029 Madrid, Spain * Correspondence: [email protected]; Tel.: +34-915-622-900 Received: 18 September 2019; Accepted: 7 October 2019; Published: 9 October 2019 Abstract: Bacterial, protozoan and other microbial infections share an accelerated metabolic rate. In order to ensure a proper functioning of cell replication and proteins and nucleic acids synthesis processes, folate metabolism rate is also increased in these cases. For this reason, folic acid antagonists have been used since their discovery to treat different kinds of microbial infections, taking advantage of this metabolic difference when compared with human cells. However, resistances to these compounds have emerged since then and only combined therapies are currently used in clinic. In addition, some of these compounds have been found to have an immunomodulatory behavior that allows clinicians using them as anti-inflammatory or immunosuppressive drugs. Therefore, the aim of this review is to provide an updated state-of-the-art on the use of antifolates as antibacterial and immunomodulating agents in the clinical setting, as well as to present their action mechanisms and currently investigated biomedical applications. Keywords: folic acid antagonists; antifolates; antibiotics; antibacterials; immunomodulation; sulfonamides; antimalarial 1. -

One Carbon Metabolism and Its Clinical Significance
One carbon metabolism and its clinical significance Dr. Kiran Meena Department of Biochemistry Class 5 : 10-10-2019 (8:00 to 9:00 AM ) Specific Learning Objectives Describe roles of folic acid, cobalamin and S-adenosylmethionine (SAM) in transfer of one carbon units between molecules, and apply their relevance to disease states Describe synthesis of S-adenosylmethionine and its role in methylation reactions Explain how a cobalamin deficiency leads to a secondary folate deficiency Introduction Human body cannot synthesize folic acid Its also called vitamin B9 give rise to tetrahydrofolate (THF), carry one carbon groups ex. Methyl group Intestines releases mostly N5-methy-THF into blood One-carbon (1C) metabolism, mediated by folate cofactor, supports biosynthesis of purines and pyrimidines, aa homeostasis (glycine, serine and methionine) Table 14.4: DM Vasudevan’ s Textbook of Biochemistry for Medical Students 6th edition Enzyme co-factors involved in aa catabolism Involves one of three co-factors: Biotin, Tetrahydrofolate (THF) and S- adenosylmethionine (SAM) These cofactors transfer one-carbon groups in different oxidation states: 1. Biotin transfers carbon in its most oxidized state CO2, it require for catabolism and utilization of branched chain aa • Biotin responsible for carbon dioxide transfer in several carboxylase enzymes Cont-- 2. Tetrahydrofolate (THF) transfers one-carbon groups in intermediate oxidation states and as methyl groups • Tetrahydrobiopterin (BH4, THB) is a cofactor of degradation of phenylalanine • Oxidised form of THF, folate is vitamin for mammals • It converted into THF by DHF reductase 3. S-adenosylmethionine (SAM) transfers methyl groups, most reduced state of carbon THF and SAM imp in aa and nucleotide metabolism SAM used in biosynthesis of creatine, phosphatidylcholine, plasmenylcholine and epinephrine, also methylated DNA, RNA and proteins Enzymes use cobalamin as a cofactor 1. -
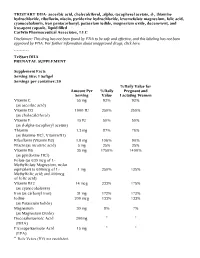
TRISTART DHA- Ascorbic Acid, Cholecalciferol, .Alpha
TRISTART DHA- ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, d-, thiamine hydrochloride, riboflavin, niacin, pyridoxine hydrochloride, levomefolate magnesium, folic acid, cyanocobalamin, iron pentacarbonyl, potassium iodide, magnesium oxide, doconexent, and icosapent capsule, liquid filled CarWin Pharmaceutical Associates, LLC Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here. ---------- TriStart DHA PRENATAL SUPPLEMENT Supplement Facts Serving Size: 1 Softgel Servings per container: 30 %Daily Value for Amount Per %Daily Pregnant and Serving Value Lactating Women Vitamin C 55 mg 92% 92% (as ascorbic acid) Vitamin D3 1000 IU 250% 250% (as cholecalciferol) Vitamin E 15 IU 50% 50% (as d-alpha-tocopheryl acetate) Thiamin 1.3 mg 87% 76% (as thiamine HCl, Vitamin B1) Riboflavin (Vitamin B2) 1.8 mg 106% 90% Niacin (as nicotinic acid) 5 mg 25% 25% Vitamin B6 35 mg 1750% 1400% (as pyridoxine HCl) Folate (as 630 mcg of L- Methylfolate Magnesium, molar equivalent to 600mcg of L- 1 mg 250% 125% Methylfolic acid; and 400mcg of folic acid) Vitamin B12 14 mcg 233% 175% (as cyanocobalamin) Iron (as carbonyl iron) 31 mg 172% 172% Iodine 200 mcg 133% 133% (as Potassium Iodide) Magnesium 30 mg 8% 7% (as Magnesium Oxide) Docosahexaenoic Acid 200mg * * (DHA) Eicosapentaenoate Acid 15 mg * * (EPA) * Daily Values (DV) not established. OTHER INGREDIENTS: Gelatin (bovine), Glycerin, Purified Water, Yellow Bees Wax, Caramel Powder, Soy Lecithin, Natural Orange Flavor, Ethyl Vanillin. Contains: Soy and Fish TriStart DHA™ Softgel capsules are dye free, lactose, gluten and sugar free. -

L-Carnitine, Mecobalamin and Folic Acid Tablets) TRINERVE-LC
For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only (L-Carnitine, Mecobalamin and Folic acid Tablets) TRINERVE-LC 1. Name of the medicinal product Trinerve-LC Tablets 2. Qualitative and quantitative composition Each film- coated tablets contains L-Carnitine…………………….500 mg Mecobalamin……………….1500 mcg Folic acid IP…………………..1.5mg 3. Pharmaceutical form Film- coated tablets 4. Clinical particulars 4.1 Therapeutic indications Vitamin and micronutrient supplementation in the management of chronic disease. 4.2 Posology and method of administration For oral administration only. One tablet daily or as directed by physician. 4.3 Contraindications Hypersensitivity to any constituent of the product. 4.4 Special warnings and precautions for use L-Carnitine The safety and efficacy of oral L-Carnitine has not been evaluated in patients with renal insufficiency. Chronic administration of high doses of oral L-Carnitine in patients with severely compromised renal function or in ESRD patients on dialysis may result in accumulation of the potentially toxic metabolites, trimethylamine (TMA) and trimethylamine-N-oxide (TMAO), since these metabolites are normally excreted in the urine. Mecobalamin Should be given with caution in patients suffering from folate deficiency. The following warnings and precautions suggested with parent form – vitamin B12 The treatment of vitamin B12 deficiency can unmask the symptoms of polycythemia vera. Megaloblastic anemia is sometimes corrected by treatment with vitamin B12. But this can have very serious side effects. Don’t attempt vitamin B12 therapy without close supervision by healthcare provider. Do not take vitamin B12 if Leber’s disease, a hereditary eye disease. -

Nutrition 102 – Class 3
Nutrition 102 – Class 3 Angel Woolever, RD, CD 1 Nutrition 102 “Introduction to Human Nutrition” second edition Edited by Michael J. Gibney, Susan A. Lanham-New, Aedin Cassidy, and Hester H. Vorster May be purchased online but is not required for the class. 2 Technical Difficulties Contact: Erin Deichman 574.753.1706 [email protected] 3 Questions You may raise your hand and type your question. All questions will be answered at the end of the webinar to save time. 4 Review from Last Week Vitamins E, K, and C What it is Source Function Requirement Absorption Deficiency Toxicity Non-essential compounds Bioflavonoids: Carnitine, Choline, Inositol, Taurine, and Ubiquinone Phytoceuticals 5 Priorities for Today’s Session B Vitamins What they are Source Function Requirement Absorption Deficiency Toxicity 6 7 What Is Vitamin B1 First B Vitamin to be discovered 8 Vitamin B1 Sources Pork – rich source Potatoes Whole-grain cereals Meat Fish 9 Functions of Vitamin B1 Converts carbohydrates into glucose for energy metabolism Strengthens immune system Improves body’s ability to withstand stressful conditions 10 Thiamine Requirements Groups: RDA (mg/day): Infants 0.4 Children 0.7-1.2 Males 1.5 Females 1 Pregnancy 2 Lactation 2 11 Thiamine Absorption Absorbed in the duodenum and proximal jejunum Alcoholics are especially susceptible to thiamine deficiency Excreted in urine, diuresis, and sweat Little storage of thiamine in the body 12 Barriers to Thiamine Absorption Lost into cooking water Unstable to light Exposure to sunlight Destroyed -

(12) Patent Application Publication (10) Pub. No.: US 2005/0196469 A1 Thys-Jacobs (43) Pub
US 2005O196469A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0196469 A1 Thys-Jacobs (43) Pub. Date: Sep. 8, 2005 (54) MICRONUTRIENT SUPPLEMENT (22) Filed: Mar. 4, 2004 COMBINATION FOR ACNE TREATMENT AND PREVENTION Publication Classification (76) Inventor: Susan Thys-Jacobs, Larchmont, NY (51) Int. Cl.' ....................... A61 K 31/59; A61 K 31/525; (US) A61K 33/10; A61K 31/19 (52) U.S. Cl. ......................... 424/687; 514/168; 514/251; Correspondence Address: 514/574 GOTTLEB RACKMAN & REISMAN PC 27O MADSON AVENUE 8TH FLOOR (57) ABSTRACT NEW YORK, NY 100160601 A micronutrient Supplement comprising effective amounts (21) Appl. No.: 10/794,729 of calcium, Vitamin D, and folate treats and prevents acne. US 2005/0196469 A1 Sep. 8, 2005 MICRONUTRIENT SUPPLEMENT COMBINATION therapies include benzoyl peroxide which has comedolytic FOR ACNE TREATMENT AND PREVENTION and antibacterial effects, topical antibacterials Such as eryth romycin or clindamycin, azelaic acid, tazaroc, and topical FIELD OF THE INVENTION retinoids. Acne that is resistant to topical treatment requires oral antibiotics or isotretinoin. Indications for isotretinoin 0001. This invention relates to a micronutrient supple include Severe Scarring, acne that is resistant to oral antibi ment in the treatment of acne Vulgaris and inflammation. In otics and acne present for many years that quickly relapses particular, this invention relates to a multi-vitamin and when an oral antibiotic therapy is discontinued. Of note, oral mineral Supplement for improving skin and hair health. isotretinoin is a potent teratogen. Current Standards of acne therapy include the topical descquarnative drugs and antibac BACKGROUND OF THE INVENTION terial agents. -

Tall Man Lettering List REPORT DECEMBER 2013 1
Tall Man Lettering List REPORT DECEMBER 2013 1 TALL MAN LETTERING LIST REPORT WWW.HQSC.GOVT.NZ Published in December 2013 by the Health Quality & Safety Commission. This document is available on the Health Quality & Safety Commission website, www.hqsc.govt.nz ISBN: 978-0-478-38555-7 (online) Citation: Health Quality & Safety Commission. 2013. Tall Man Lettering List Report. Wellington: Health Quality & Safety Commission. Crown copyright ©. This copyright work is licensed under the Creative Commons Attribution-No Derivative Works 3.0 New Zealand licence. In essence, you are free to copy and distribute the work (including other media and formats), as long as you attribute the work to the Health Quality & Safety Commission. The work must not be adapted and other licence terms must be abided. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nd/3.0/nz/ Copyright enquiries If you are in doubt as to whether a proposed use is covered by this licence, please contact: National Medication Safety Programme Team Health Quality & Safety Commission PO Box 25496 Wellington 6146 ACKNOWLEDGEMENTS The Health Quality & Safety Commission acknowledges the following for their assistance in producing the New Zealand Tall Man lettering list: • The Australian Commission on Safety and Quality in Health Care for advice and support in allowing its original work to be either reproduced in whole or altered in part for New Zealand as per its copyright1 • The Medication Safety and Quality Program of Clinical Excellence Commission, New South -

BC Cancer Benefit Drug List September 2021
Page 1 of 65 BC Cancer Benefit Drug List September 2021 DEFINITIONS Class I Reimbursed for active cancer or approved treatment or approved indication only. Reimbursed for approved indications only. Completion of the BC Cancer Compassionate Access Program Application (formerly Undesignated Indication Form) is necessary to Restricted Funding (R) provide the appropriate clinical information for each patient. NOTES 1. BC Cancer will reimburse, to the Communities Oncology Network hospital pharmacy, the actual acquisition cost of a Benefit Drug, up to the maximum price as determined by BC Cancer, based on the current brand and contract price. Please contact the OSCAR Hotline at 1-888-355-0355 if more information is required. 2. Not Otherwise Specified (NOS) code only applicable to Class I drugs where indicated. 3. Intrahepatic use of chemotherapy drugs is not reimbursable unless specified. 4. For queries regarding other indications not specified, please contact the BC Cancer Compassionate Access Program Office at 604.877.6000 x 6277 or [email protected] DOSAGE TUMOUR PROTOCOL DRUG APPROVED INDICATIONS CLASS NOTES FORM SITE CODES Therapy for Metastatic Castration-Sensitive Prostate Cancer using abiraterone tablet Genitourinary UGUMCSPABI* R Abiraterone and Prednisone Palliative Therapy for Metastatic Castration Resistant Prostate Cancer abiraterone tablet Genitourinary UGUPABI R Using Abiraterone and prednisone acitretin capsule Lymphoma reversal of early dysplastic and neoplastic stem changes LYNOS I first-line treatment of epidermal -

Nutritional Interventions for Autism Spectrum Disorder
Lead Article Nutritional interventions for autism spectrum disorder Downloaded from https://academic.oup.com/nutritionreviews/advance-article-abstract/doi/10.1093/nutrit/nuz092/5687289 by Florida Atlantic University user on 06 January 2020 Elisa Karhu*, Ryan Zukerman*, Rebecca S. Eshraghi, Jeenu Mittal, Richard C. Deth, Ana M. Castejon, Malav Trivedi, Rahul Mittal, and Adrien A. Eshraghi Autism spectrum disorder (ASD) is an increasingly prevalent neurodevelopmental dis- order with considerable clinical heterogeneity. With no cure for the disorder, treat- ments commonly center around speech and behavioral therapies to improve the characteristic social, behavioral, and communicative symptoms of ASD. Gastrointestinal disturbances are commonly encountered comorbidities that are thought to be not only another symptom of ASD but to also play an active role in modulating the expression of social and behavioral symptoms. Therefore, nutritional interventions are used by a majority of those with ASD both with and without clinical supervision to alleviate gastrointestinal and behavioral symptoms. Despite a consider- able interest in dietary interventions, no consensus exists regarding optimal nutritional therapy. Thus, patients and physicians are left to choose from a myriad of dietary pro- tocols. This review, summarizes the state of the current clinical and experimental liter- ature on nutritional interventions for ASD, including gluten-free and casein-free, keto- genic, and specific carbohydrate diets, as well as probiotics, polyunsaturated fatty -

B-COMPLEX FORTE with VITAMIN C CAPSULES BECOSULES Capsules
For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory. B-COMPLEX FORTE WITH VITAMIN C CAPSULES BECOSULES Capsules 1. NAME OF THE MEDICINAL PRODUCT BECOSULES 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains: Thiamine Mononitrate I.P. 10 mg Riboflavin I.P. 10 mg Pyridoxine Hydrochloride I.P. 3 mg Vitamin B12 I.P. ( as STABLETS 1:100) 15 mcg Niacinamide I.P. 100 mg Calcium Pantothenate I.P. 50 mg Folic Acid I.P. 1.5 mg Biotin U.S.P. 100 mcg Ascorbic Acid I.P. (as coated) 150 mg Appropriate overages added For Therapeutic Use For a full list of excipients, see section 6.1. All strengths/presentations mentioned in this document might not be available in the market. 3. PHARMACOLOGICAL FORM Capsules 4. CLINICAL PARTICULARS 4.1 Therapeutic Indications Trademark Proprietor: Pfizer Products Inc. USA Licensed User: Pfizer Limited, India BECOSULES Capsules Page 1 of 7 LPDBCC092017 PfLEET Number: 2017-0033507 Becosules capsules are indicated in the treatment of patients with deficiencies of, or increased requirement for, vitamin B-complex, and vitamin C. Such patients and conditions include: Decreased intake because of restricted or unbalanced diet as in anorexia, diabetes mellitus, obesity and alcoholism. Reduced availability during treatment with antimicrobials which alter normal intestinal flora, in prolonged diarrhea and in chronic gastro-intestinal disorders. Increased requirements due to increased metabolic rate as in fever and tissue wasting, e.g. febrile illness, acute or chronic infections, surgery, burns and fractures. Stomatitis, glossitis, cheilosis, paraesthesias, neuralgia and dermatitis. Micronutrient deficiencies during pregnancy or lactation. -

5,10-Methylenetetrahydrofolate Dehydrogenase (Ec 1.5.1.5)
Enzymatic Assay of 5,10-METHYLENETETRAHYDROFOLATE DEHYDROGENASE (EC 1.5.1.5) PRINCIPLE: 5,10-MeFH4DH 5,10-Methylene-FH4 + ß-NADP > 5,10-Methenyl-FH4 + ß-NADPH Abbreviations used: 5,10-Methylene-FH4 = 5,10-Methylenetetrahydrofolate 5,10-Methenyl-FH4 = 5,10-Methenyltetrahydrofolate 5,10-MeFH4DH = 5,10-Methylenetetrahydrofolate Dehydrogenase ß-NADP = ß-Nicotinamide Adenine Dinucleotide Phosphate, Oxidized Form ß-NADPH = ß-Nicotinamide Adenine Dinucleotide Phosphate, Reduced Form CONDITIONS: T = 25°C, pH = 7.5, A340nm, Light path = 1 cm METHOD: Continuous Spectrophotometric Rate Determination REAGENTS: A. 50 mM Potassium Phosphate Buffer with 100 mM Potassium Chloride, pH 7.5 at 25°C (Prepare 200 ml in deionized water using Potassium Phosphate, Monobasic, Anhydrous, and Potassium Chloride. Adjust to pH 7.5 at 25°C with 1 M NaOH.) B. 0.002% (w/v) Tetrahydrofolic Acid with 0.002% (v/v) Formaldehyde and 0.1% (v/v) 2-Mercaptoethanol Solution (FH4) (Immediately before used, prepare 100 ml in Reagent A using Tetrahydrofolic Acid, Formaldehyde, 37% Solution,1 and 2-Mercaptoethanol. ) C. 20 mM ß-Nicotinamide Adenine Dinucleotide Phosphate (ß-NADP) (Prepare 2 ml in deionized water using ß-Nicotinamide Adenine Dinucleotide Phosphate, Sodium Salt PREPARE FRESH. ) Page 1 of 3 45-1 Ramsey Road, Shirley, NY 11967, USA Email: [email protected] Tel: 1-631-562-8517 1-631-448-7888 Fax: 1-631-938-8127 Enzymatic Assay of 5,10-METHYLENETETRAHYDROFOLATE DEHYDROGENASE (EC 1.5.1.5) REAGENTS: (continued) D. 5,10-Methylenetetrahydrofolate Dehydrogenase Enzyme Solution (Immediately before use, prepare a solution containing 0.01 - 0.03 unit/ml of 5,10-Methylenetetrahydrofolate Dehydrogenase in cold Reagent A.) PROCEDURE: Pipette (in milliliters) the following reagents into suitable cuvettes: Test Blank Reagent B (FH4) 2.80 2.80 Reagent D (Enzyme Solution) 0.10 0.10 Mix by inversion and equilibrate to 25°C. -

5,10-Methylenetetrahydrofolate Dehydrogenasefrom
JOURNAL OF BACTERIOLOGY, Feb. 1991, p. 1414-1419 Vol. 173, No. 4 0021-9193/91/041414-06$02.00/0 Copyright 0 1991, American Society for Microbiology Purification and Characterization of NADP+-Dependent 5,10-Methylenetetrahydrofolate Dehydrogenase from Peptostreptococcus productus Marburg GERT WOHLFARTH, GABRIELE GEERLIGS, AND GABRIELE DIEKERT* Institutfur Mikrobiologie, Universitat Stuttgart, Azenbergstrasse 18, D-7000 Stuttgart 1, Federal Republic of Germany Received 19 June 1990/Accepted 7 December 1990 The 5,10-methylenetetrahydrofolate dehydrogenase of heterotrophicaily grown Peptostreptococcus productus Marburg was purified to apparent homogeneity. The purified enzyme catalyzed the reversible oxidation of methylenetetrahydrofolate with NADP+ as the electron acceptor at a specific activity of 627 U/mg of protein. The Km values for methylenetetrahydrofolate and for NADP+ were 27 and 113 ,M, respectively. The enzyme, which lacked 5,10-methenyltetrahydrofolate cyclohydrolase activity, was insensitive to oxygen and was thermolabile at temperatures above 40C. The apparent molecular mass of the enzyme was estimated by gel filtration to be 66 kDa. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed the presence of a single subunit of 34 kDa, accounting for a dimeric a2 structure of the enzyme. Kinetic studies on the initial reaction velocities with different concentrations of both substrates in the absence and presence of NADPH as the reaction product were interpreted to indicate that the enzyme followed a sequential reaction mechanism. After gentle ultracentrifugation of crude extracts, the enzyme was recovered to >95% in the soluble (supernatant) fraction. Sodium (10 FM to 10 mM) had no effect on enzymatic activity. The data were taken to indicate that the enzyme was similar to the methylenetetrahydrofolate dehydrogenases of other homoacetogenic bacteria and that the enzyme is not involved in energy conservation of P.