Enlyte®With Deltafolatetm
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

One Carbon Metabolism and Its Clinical Significance
One carbon metabolism and its clinical significance Dr. Kiran Meena Department of Biochemistry Class 5 : 10-10-2019 (8:00 to 9:00 AM ) Specific Learning Objectives Describe roles of folic acid, cobalamin and S-adenosylmethionine (SAM) in transfer of one carbon units between molecules, and apply their relevance to disease states Describe synthesis of S-adenosylmethionine and its role in methylation reactions Explain how a cobalamin deficiency leads to a secondary folate deficiency Introduction Human body cannot synthesize folic acid Its also called vitamin B9 give rise to tetrahydrofolate (THF), carry one carbon groups ex. Methyl group Intestines releases mostly N5-methy-THF into blood One-carbon (1C) metabolism, mediated by folate cofactor, supports biosynthesis of purines and pyrimidines, aa homeostasis (glycine, serine and methionine) Table 14.4: DM Vasudevan’ s Textbook of Biochemistry for Medical Students 6th edition Enzyme co-factors involved in aa catabolism Involves one of three co-factors: Biotin, Tetrahydrofolate (THF) and S- adenosylmethionine (SAM) These cofactors transfer one-carbon groups in different oxidation states: 1. Biotin transfers carbon in its most oxidized state CO2, it require for catabolism and utilization of branched chain aa • Biotin responsible for carbon dioxide transfer in several carboxylase enzymes Cont-- 2. Tetrahydrofolate (THF) transfers one-carbon groups in intermediate oxidation states and as methyl groups • Tetrahydrobiopterin (BH4, THB) is a cofactor of degradation of phenylalanine • Oxidised form of THF, folate is vitamin for mammals • It converted into THF by DHF reductase 3. S-adenosylmethionine (SAM) transfers methyl groups, most reduced state of carbon THF and SAM imp in aa and nucleotide metabolism SAM used in biosynthesis of creatine, phosphatidylcholine, plasmenylcholine and epinephrine, also methylated DNA, RNA and proteins Enzymes use cobalamin as a cofactor 1. -
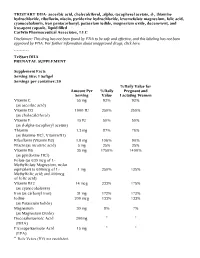
TRISTART DHA- Ascorbic Acid, Cholecalciferol, .Alpha
TRISTART DHA- ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, d-, thiamine hydrochloride, riboflavin, niacin, pyridoxine hydrochloride, levomefolate magnesium, folic acid, cyanocobalamin, iron pentacarbonyl, potassium iodide, magnesium oxide, doconexent, and icosapent capsule, liquid filled CarWin Pharmaceutical Associates, LLC Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here. ---------- TriStart DHA PRENATAL SUPPLEMENT Supplement Facts Serving Size: 1 Softgel Servings per container: 30 %Daily Value for Amount Per %Daily Pregnant and Serving Value Lactating Women Vitamin C 55 mg 92% 92% (as ascorbic acid) Vitamin D3 1000 IU 250% 250% (as cholecalciferol) Vitamin E 15 IU 50% 50% (as d-alpha-tocopheryl acetate) Thiamin 1.3 mg 87% 76% (as thiamine HCl, Vitamin B1) Riboflavin (Vitamin B2) 1.8 mg 106% 90% Niacin (as nicotinic acid) 5 mg 25% 25% Vitamin B6 35 mg 1750% 1400% (as pyridoxine HCl) Folate (as 630 mcg of L- Methylfolate Magnesium, molar equivalent to 600mcg of L- 1 mg 250% 125% Methylfolic acid; and 400mcg of folic acid) Vitamin B12 14 mcg 233% 175% (as cyanocobalamin) Iron (as carbonyl iron) 31 mg 172% 172% Iodine 200 mcg 133% 133% (as Potassium Iodide) Magnesium 30 mg 8% 7% (as Magnesium Oxide) Docosahexaenoic Acid 200mg * * (DHA) Eicosapentaenoate Acid 15 mg * * (EPA) * Daily Values (DV) not established. OTHER INGREDIENTS: Gelatin (bovine), Glycerin, Purified Water, Yellow Bees Wax, Caramel Powder, Soy Lecithin, Natural Orange Flavor, Ethyl Vanillin. Contains: Soy and Fish TriStart DHA™ Softgel capsules are dye free, lactose, gluten and sugar free. -

Mobile Loop Dynamics in Adenosyltransferase Control
Mobile loop dynamics in adenosyltransferase control binding and reactivity of coenzyme B12 Romila Mascarenhasa,1, Markus Ruetza,1, Liam McDevitta, Markos Koutmosb,c, and Ruma Banerjeea,2 aDepartment of Biological Chemistry, University of Michigan, Ann Arbor, MI 48109-0600; bDepartment of Chemistry, University of Michigan, Ann Arbor, MI 48109-0600, and cDepartment of Biophysics, University of Michigan, Ann Arbor, MI 48109-0600 Edited by Amie K. Boal, Pennsylvania State University, State College, PA, and accepted by Editorial Board Member Stephen J. Benkovic October 20, 2020 (received for review April 16, 2020) Cobalamin is a complex organometallic cofactor that is processed Human and Methylobacterium extorquens (Me) ATR, which have and targeted via a network of chaperones to its dependent been characterized most extensively, also double as chaperones, enzymes. AdoCbl (5′-deoxyadenosylcobalamin) is synthesized transferring AdoCbl directly to MCM (Fig. 1A) rather than re- from cob(II)alamin in a reductive adenosylation reaction catalyzed leasing the high-value product into solution (12–17). Cofactor by adenosyltransferase (ATR), which also serves as an escort, de- loading onto MCM is gated by the GTPase activity of the G livering AdoCbl to methylmalonyl-CoA mutase (MCM). The mech- protein chaperone CblA (18). Despite the overall structural and anism by which ATR signals that its cofactor cargo is ready functional conservation of the cofactor loading processes, there (AdoCbl) or not [cob(II)alamin] for transfer to MCM, is not known. are important differences in regulation between the human (19) In this study, we have obtained crystallographic snapshots that – reveal ligand-induced ordering of the N terminus of Mycobacte- and the better-characterized bacterial systems (15 17). -

Nutrition 102 – Class 3
Nutrition 102 – Class 3 Angel Woolever, RD, CD 1 Nutrition 102 “Introduction to Human Nutrition” second edition Edited by Michael J. Gibney, Susan A. Lanham-New, Aedin Cassidy, and Hester H. Vorster May be purchased online but is not required for the class. 2 Technical Difficulties Contact: Erin Deichman 574.753.1706 [email protected] 3 Questions You may raise your hand and type your question. All questions will be answered at the end of the webinar to save time. 4 Review from Last Week Vitamins E, K, and C What it is Source Function Requirement Absorption Deficiency Toxicity Non-essential compounds Bioflavonoids: Carnitine, Choline, Inositol, Taurine, and Ubiquinone Phytoceuticals 5 Priorities for Today’s Session B Vitamins What they are Source Function Requirement Absorption Deficiency Toxicity 6 7 What Is Vitamin B1 First B Vitamin to be discovered 8 Vitamin B1 Sources Pork – rich source Potatoes Whole-grain cereals Meat Fish 9 Functions of Vitamin B1 Converts carbohydrates into glucose for energy metabolism Strengthens immune system Improves body’s ability to withstand stressful conditions 10 Thiamine Requirements Groups: RDA (mg/day): Infants 0.4 Children 0.7-1.2 Males 1.5 Females 1 Pregnancy 2 Lactation 2 11 Thiamine Absorption Absorbed in the duodenum and proximal jejunum Alcoholics are especially susceptible to thiamine deficiency Excreted in urine, diuresis, and sweat Little storage of thiamine in the body 12 Barriers to Thiamine Absorption Lost into cooking water Unstable to light Exposure to sunlight Destroyed -

(12) Patent Application Publication (10) Pub. No.: US 2005/0196469 A1 Thys-Jacobs (43) Pub
US 2005O196469A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0196469 A1 Thys-Jacobs (43) Pub. Date: Sep. 8, 2005 (54) MICRONUTRIENT SUPPLEMENT (22) Filed: Mar. 4, 2004 COMBINATION FOR ACNE TREATMENT AND PREVENTION Publication Classification (76) Inventor: Susan Thys-Jacobs, Larchmont, NY (51) Int. Cl.' ....................... A61 K 31/59; A61 K 31/525; (US) A61K 33/10; A61K 31/19 (52) U.S. Cl. ......................... 424/687; 514/168; 514/251; Correspondence Address: 514/574 GOTTLEB RACKMAN & REISMAN PC 27O MADSON AVENUE 8TH FLOOR (57) ABSTRACT NEW YORK, NY 100160601 A micronutrient Supplement comprising effective amounts (21) Appl. No.: 10/794,729 of calcium, Vitamin D, and folate treats and prevents acne. US 2005/0196469 A1 Sep. 8, 2005 MICRONUTRIENT SUPPLEMENT COMBINATION therapies include benzoyl peroxide which has comedolytic FOR ACNE TREATMENT AND PREVENTION and antibacterial effects, topical antibacterials Such as eryth romycin or clindamycin, azelaic acid, tazaroc, and topical FIELD OF THE INVENTION retinoids. Acne that is resistant to topical treatment requires oral antibiotics or isotretinoin. Indications for isotretinoin 0001. This invention relates to a micronutrient supple include Severe Scarring, acne that is resistant to oral antibi ment in the treatment of acne Vulgaris and inflammation. In otics and acne present for many years that quickly relapses particular, this invention relates to a multi-vitamin and when an oral antibiotic therapy is discontinued. Of note, oral mineral Supplement for improving skin and hair health. isotretinoin is a potent teratogen. Current Standards of acne therapy include the topical descquarnative drugs and antibac BACKGROUND OF THE INVENTION terial agents. -

Tall Man Lettering List REPORT DECEMBER 2013 1
Tall Man Lettering List REPORT DECEMBER 2013 1 TALL MAN LETTERING LIST REPORT WWW.HQSC.GOVT.NZ Published in December 2013 by the Health Quality & Safety Commission. This document is available on the Health Quality & Safety Commission website, www.hqsc.govt.nz ISBN: 978-0-478-38555-7 (online) Citation: Health Quality & Safety Commission. 2013. Tall Man Lettering List Report. Wellington: Health Quality & Safety Commission. Crown copyright ©. This copyright work is licensed under the Creative Commons Attribution-No Derivative Works 3.0 New Zealand licence. In essence, you are free to copy and distribute the work (including other media and formats), as long as you attribute the work to the Health Quality & Safety Commission. The work must not be adapted and other licence terms must be abided. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nd/3.0/nz/ Copyright enquiries If you are in doubt as to whether a proposed use is covered by this licence, please contact: National Medication Safety Programme Team Health Quality & Safety Commission PO Box 25496 Wellington 6146 ACKNOWLEDGEMENTS The Health Quality & Safety Commission acknowledges the following for their assistance in producing the New Zealand Tall Man lettering list: • The Australian Commission on Safety and Quality in Health Care for advice and support in allowing its original work to be either reproduced in whole or altered in part for New Zealand as per its copyright1 • The Medication Safety and Quality Program of Clinical Excellence Commission, New South -

BC Cancer Benefit Drug List September 2021
Page 1 of 65 BC Cancer Benefit Drug List September 2021 DEFINITIONS Class I Reimbursed for active cancer or approved treatment or approved indication only. Reimbursed for approved indications only. Completion of the BC Cancer Compassionate Access Program Application (formerly Undesignated Indication Form) is necessary to Restricted Funding (R) provide the appropriate clinical information for each patient. NOTES 1. BC Cancer will reimburse, to the Communities Oncology Network hospital pharmacy, the actual acquisition cost of a Benefit Drug, up to the maximum price as determined by BC Cancer, based on the current brand and contract price. Please contact the OSCAR Hotline at 1-888-355-0355 if more information is required. 2. Not Otherwise Specified (NOS) code only applicable to Class I drugs where indicated. 3. Intrahepatic use of chemotherapy drugs is not reimbursable unless specified. 4. For queries regarding other indications not specified, please contact the BC Cancer Compassionate Access Program Office at 604.877.6000 x 6277 or [email protected] DOSAGE TUMOUR PROTOCOL DRUG APPROVED INDICATIONS CLASS NOTES FORM SITE CODES Therapy for Metastatic Castration-Sensitive Prostate Cancer using abiraterone tablet Genitourinary UGUMCSPABI* R Abiraterone and Prednisone Palliative Therapy for Metastatic Castration Resistant Prostate Cancer abiraterone tablet Genitourinary UGUPABI R Using Abiraterone and prednisone acitretin capsule Lymphoma reversal of early dysplastic and neoplastic stem changes LYNOS I first-line treatment of epidermal -

Nutritional Interventions for Autism Spectrum Disorder
Lead Article Nutritional interventions for autism spectrum disorder Downloaded from https://academic.oup.com/nutritionreviews/advance-article-abstract/doi/10.1093/nutrit/nuz092/5687289 by Florida Atlantic University user on 06 January 2020 Elisa Karhu*, Ryan Zukerman*, Rebecca S. Eshraghi, Jeenu Mittal, Richard C. Deth, Ana M. Castejon, Malav Trivedi, Rahul Mittal, and Adrien A. Eshraghi Autism spectrum disorder (ASD) is an increasingly prevalent neurodevelopmental dis- order with considerable clinical heterogeneity. With no cure for the disorder, treat- ments commonly center around speech and behavioral therapies to improve the characteristic social, behavioral, and communicative symptoms of ASD. Gastrointestinal disturbances are commonly encountered comorbidities that are thought to be not only another symptom of ASD but to also play an active role in modulating the expression of social and behavioral symptoms. Therefore, nutritional interventions are used by a majority of those with ASD both with and without clinical supervision to alleviate gastrointestinal and behavioral symptoms. Despite a consider- able interest in dietary interventions, no consensus exists regarding optimal nutritional therapy. Thus, patients and physicians are left to choose from a myriad of dietary pro- tocols. This review, summarizes the state of the current clinical and experimental liter- ature on nutritional interventions for ASD, including gluten-free and casein-free, keto- genic, and specific carbohydrate diets, as well as probiotics, polyunsaturated fatty -

Enbrace® HR DESCRIPTION: INGREDIENTS
EnBrace® HR with DeltaFolate ™ [1 NF Units] [15 mg DFE Folate] ANTI-ANEMIA PREPARATION as extrinsic/intrinsic factor concentrate plus folate. Prescription Prenatal/Vitamin Drug For Therapeutic Use Multi-phasic Capsules (30ct bottle) NDC 64661-650-30 Rx Only [DRUG] GLUTEN-FREE DESCRIPTION: EnBrace® HR is an orally administered prescription prenatal/vitamin drug for therapeutic use formulated for female macrocytic anemia patients that are in need of treatment, and are under specific direction and monitoring of vitamin B12 and vitamin B9 status by a physician. EnBrace® HR is intended for women of childbearing age who are – or desire to become, pregnant regardless of lactation status. EnBrace® HR may be prescribed for women at risk of depression as a result of folate or cobalamin deficiency - including folate-induced postpartum depression, or are at risk of folate-induced birth defects such as may be found with spina bifida and other neural tube defects (NTDs). INGREDIENTS: Cobalamin intrinsic factor complex 1 NF Units* * National Formulary Units (“NF UNITS”) equivalent to 50 mcg of active coenzyme cobalamin (as cobamamide concentrate with intrinsic factor) ALSO CONTAINS: 1 Folinic acid (B9-vitamer) 2.5 mg + 1 Control-release, citrated folic acid, DHF (B9-Provitamin) 1 mg 2 Levomefolic acid (B9 & B12- cofactor) 5.23 mg 1 6 mg DFE folate (vitamin B9) 2 9 mg DFE l-methylfolate magnesium (molar equivalent). FUNCTIONAL EXCIPIENTS: 13.6 mg FeGC as ferrous glycine cysteinate (1.5 mg 3 3,4 elemental iron ) [colorant], 25 mg ascorbates (24 mg magnesium l-ascorbate, 1 mg zinc l-ascorbate) [antioxidant], at least 23.33 mg phospholipid-omega3 complex5 [marine lipids], 500 mcg betaine (trimethylglycine) [acidifier], 1 mg magnesium l-threonate [stabilizer]. -

Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline 9 Vitamin B12
Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline http://www.nap.edu/catalog/6015.html 9 Vitamin B12 SUMMARY Vitamin B12 (cobalamin) functions as a coenzyme for a critical methyl transfer reaction that converts homocysteine to methionine and for a separate reaction that converts L-methylmalonyl- coenzyme A (CoA) to succinyl-CoA. The Recommended Dietary Allowance (RDA) for vitamin B12 is based on the amount needed for the maintenance of hematological status and normal serum vitamin B12 values. An assumed absorption of 50 percent is in- cluded in the recommended intake. The RDA for adults is 2.4 µg/ day of vitamin B12. Because 10 to 30 percent of older people may be unable to absorb naturally occurring vitamin B12, it is advisable for those older than 50 years to meet their RDA mainly by consum- ing foods fortified with vitamin B12 or a vitamin B12-containing supplement. Individuals with vitamin B12 deficiency caused by a lack of intrinsic factor require medical treatment. The median intake of vitamin B12 from food in the United States was estimated to be approximately 5 µg/day for men and 3.5 µg/day for women. The ninety-fifth percentile of vitamin B12 intake from both food and supplements was approximately 27 µg/day. In one Canadian province the mean dietary intake was estimated to be approxi- mately 7 µg/day for men and 4 µg/day for women. There is not sufficient scientific evidence to set a Tolerable Upper Intake Level (UL) for vitamin B12 at this time. -

Heterodisulfide Reductase from Methanogenic Archaea: a New Catalytic Role for an Iron-Sulfur Cluster
Article in press - uncorrected proof Biol. Chem., Vol. 386, pp. 961–970, October 2005 • Copyright ᮊ by Walter de Gruyter • Berlin • New York. DOI 10.1515/BC.2005.112 Review Heterodisulfide reductase from methanogenic archaea: a new catalytic role for an iron-sulfur cluster Reiner Hedderich1,*, Nils Hamann1 and Marina (for a recent review see Walters and Johnson, 2004). Bennati2 Data obtained with both enzymes strongly indicate that the role of the w4Fe-4Sx cluster is to mediate electron 1 Max-Planck-Institute for Terrestrial Microbiology, transfer to the substrate disulfide and to stabilize the for- Karl-von-Frisch Strasse, D-35043 Marburg, Germany mal thiyl radical that results from initial one-electron 2 Institute of Physical and Theoretical Chemistry and reduction of the disulfide via reduction and coordination Center for Biomolecular Magnetic Resonance, J.W. q of one thiol to a site on the resultant oxidized w4Fe-4Sx3 Goethe University of Frankfurt, D-60439 Frankfurt/Main, cluster. HDR and FTR represent evolutionarily distinct Germany enzymes. They are not sequence-related and share no * Corresponding author sequence similarity with enzymes belonging to the well- e-mail: [email protected] characterized family of pyridinenucleotide disulfide oxi- doreductases. These latter enzymes catalyze the electron transfer between NAD(P)(H) and a disulfide/thiol (Wil- liams, 1995; Williams et al., 2000). Thioredoxin reductase, Abstract glutathione reductase and lipoamide dehydrogenase are Heterodisulfide reductase (HDR) from methanogenic prominent members of this enzyme family. These archaea is an iron-sulfur protein that catalyzes reversible enzymes are homodimeric flavoenzymes, having a reduction of the heterodisulfide (CoM-S-S-CoB) of the redox-active disulfide and a FAD in each monomer. -

Levomefolic Acid | Medchemexpress
Inhibitors Product Data Sheet Levomefolic acid • Agonists Cat. No.: HY-14781 CAS No.: 31690-09-2 Molecular Formula: C₂₀H₂₅N₇O₆ • Molecular Weight: 459.46 Screening Libraries Target: Endogenous Metabolite; Reactive Oxygen Species Pathway: Metabolic Enzyme/Protease; Immunology/Inflammation; NF-κB Storage: Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month SOLVENT & SOLUBILITY In Vitro H2O : 5 mg/mL (10.88 mM; Need ultrasonic) DMSO : < 1 mg/mL (insoluble or slightly soluble) Mass Solvent 1 mg 5 mg 10 mg Concentration Preparing 1 mM 2.1765 mL 10.8823 mL 21.7647 mL Stock Solutions 5 mM 0.4353 mL 2.1765 mL 4.3529 mL 10 mM 0.2176 mL 1.0882 mL 2.1765 mL Please refer to the solubility information to select the appropriate solvent. BIOLOGICAL ACTIVITY Description Levomefolic acid (5-MTHF) is the natural, active form of folic acid used at the cellular level for DNA reproduction, the cysteine cycle and the regulation of homocysteine among other functions. IC50 value: Target: Folate analogLevomefolic acid has been proposed for treatment of cardiovascular disease and advanced cancers such as breast and colorectal cancers. Levomefolic acid (5-MTHF) has the prominent antioxidant activity. A high dose of 5-MTHF or folic acid does not influence Natural killer (NK) cell function in vitro. CUSTOMER VALIDATION • J Mol Med (Berl). 2019 Aug;97(8):1183-1193. See more customer validations on www.MedChemExpress.com Page 1 of 2 www.MedChemExpress.com REFERENCES [1]. Hirsch S, et al. Natural killer cell cytotoxicity is not regulated by folic acid in vitro.