Submission for Folic Acid
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
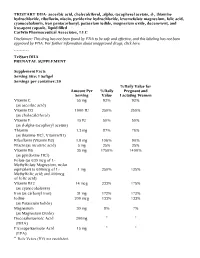
TRISTART DHA- Ascorbic Acid, Cholecalciferol, .Alpha
TRISTART DHA- ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, d-, thiamine hydrochloride, riboflavin, niacin, pyridoxine hydrochloride, levomefolate magnesium, folic acid, cyanocobalamin, iron pentacarbonyl, potassium iodide, magnesium oxide, doconexent, and icosapent capsule, liquid filled CarWin Pharmaceutical Associates, LLC Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here. ---------- TriStart DHA PRENATAL SUPPLEMENT Supplement Facts Serving Size: 1 Softgel Servings per container: 30 %Daily Value for Amount Per %Daily Pregnant and Serving Value Lactating Women Vitamin C 55 mg 92% 92% (as ascorbic acid) Vitamin D3 1000 IU 250% 250% (as cholecalciferol) Vitamin E 15 IU 50% 50% (as d-alpha-tocopheryl acetate) Thiamin 1.3 mg 87% 76% (as thiamine HCl, Vitamin B1) Riboflavin (Vitamin B2) 1.8 mg 106% 90% Niacin (as nicotinic acid) 5 mg 25% 25% Vitamin B6 35 mg 1750% 1400% (as pyridoxine HCl) Folate (as 630 mcg of L- Methylfolate Magnesium, molar equivalent to 600mcg of L- 1 mg 250% 125% Methylfolic acid; and 400mcg of folic acid) Vitamin B12 14 mcg 233% 175% (as cyanocobalamin) Iron (as carbonyl iron) 31 mg 172% 172% Iodine 200 mcg 133% 133% (as Potassium Iodide) Magnesium 30 mg 8% 7% (as Magnesium Oxide) Docosahexaenoic Acid 200mg * * (DHA) Eicosapentaenoate Acid 15 mg * * (EPA) * Daily Values (DV) not established. OTHER INGREDIENTS: Gelatin (bovine), Glycerin, Purified Water, Yellow Bees Wax, Caramel Powder, Soy Lecithin, Natural Orange Flavor, Ethyl Vanillin. Contains: Soy and Fish TriStart DHA™ Softgel capsules are dye free, lactose, gluten and sugar free. -

Nutrition 102 – Class 3
Nutrition 102 – Class 3 Angel Woolever, RD, CD 1 Nutrition 102 “Introduction to Human Nutrition” second edition Edited by Michael J. Gibney, Susan A. Lanham-New, Aedin Cassidy, and Hester H. Vorster May be purchased online but is not required for the class. 2 Technical Difficulties Contact: Erin Deichman 574.753.1706 [email protected] 3 Questions You may raise your hand and type your question. All questions will be answered at the end of the webinar to save time. 4 Review from Last Week Vitamins E, K, and C What it is Source Function Requirement Absorption Deficiency Toxicity Non-essential compounds Bioflavonoids: Carnitine, Choline, Inositol, Taurine, and Ubiquinone Phytoceuticals 5 Priorities for Today’s Session B Vitamins What they are Source Function Requirement Absorption Deficiency Toxicity 6 7 What Is Vitamin B1 First B Vitamin to be discovered 8 Vitamin B1 Sources Pork – rich source Potatoes Whole-grain cereals Meat Fish 9 Functions of Vitamin B1 Converts carbohydrates into glucose for energy metabolism Strengthens immune system Improves body’s ability to withstand stressful conditions 10 Thiamine Requirements Groups: RDA (mg/day): Infants 0.4 Children 0.7-1.2 Males 1.5 Females 1 Pregnancy 2 Lactation 2 11 Thiamine Absorption Absorbed in the duodenum and proximal jejunum Alcoholics are especially susceptible to thiamine deficiency Excreted in urine, diuresis, and sweat Little storage of thiamine in the body 12 Barriers to Thiamine Absorption Lost into cooking water Unstable to light Exposure to sunlight Destroyed -

(12) Patent Application Publication (10) Pub. No.: US 2005/0196469 A1 Thys-Jacobs (43) Pub
US 2005O196469A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0196469 A1 Thys-Jacobs (43) Pub. Date: Sep. 8, 2005 (54) MICRONUTRIENT SUPPLEMENT (22) Filed: Mar. 4, 2004 COMBINATION FOR ACNE TREATMENT AND PREVENTION Publication Classification (76) Inventor: Susan Thys-Jacobs, Larchmont, NY (51) Int. Cl.' ....................... A61 K 31/59; A61 K 31/525; (US) A61K 33/10; A61K 31/19 (52) U.S. Cl. ......................... 424/687; 514/168; 514/251; Correspondence Address: 514/574 GOTTLEB RACKMAN & REISMAN PC 27O MADSON AVENUE 8TH FLOOR (57) ABSTRACT NEW YORK, NY 100160601 A micronutrient Supplement comprising effective amounts (21) Appl. No.: 10/794,729 of calcium, Vitamin D, and folate treats and prevents acne. US 2005/0196469 A1 Sep. 8, 2005 MICRONUTRIENT SUPPLEMENT COMBINATION therapies include benzoyl peroxide which has comedolytic FOR ACNE TREATMENT AND PREVENTION and antibacterial effects, topical antibacterials Such as eryth romycin or clindamycin, azelaic acid, tazaroc, and topical FIELD OF THE INVENTION retinoids. Acne that is resistant to topical treatment requires oral antibiotics or isotretinoin. Indications for isotretinoin 0001. This invention relates to a micronutrient supple include Severe Scarring, acne that is resistant to oral antibi ment in the treatment of acne Vulgaris and inflammation. In otics and acne present for many years that quickly relapses particular, this invention relates to a multi-vitamin and when an oral antibiotic therapy is discontinued. Of note, oral mineral Supplement for improving skin and hair health. isotretinoin is a potent teratogen. Current Standards of acne therapy include the topical descquarnative drugs and antibac BACKGROUND OF THE INVENTION terial agents. -

Tall Man Lettering List REPORT DECEMBER 2013 1
Tall Man Lettering List REPORT DECEMBER 2013 1 TALL MAN LETTERING LIST REPORT WWW.HQSC.GOVT.NZ Published in December 2013 by the Health Quality & Safety Commission. This document is available on the Health Quality & Safety Commission website, www.hqsc.govt.nz ISBN: 978-0-478-38555-7 (online) Citation: Health Quality & Safety Commission. 2013. Tall Man Lettering List Report. Wellington: Health Quality & Safety Commission. Crown copyright ©. This copyright work is licensed under the Creative Commons Attribution-No Derivative Works 3.0 New Zealand licence. In essence, you are free to copy and distribute the work (including other media and formats), as long as you attribute the work to the Health Quality & Safety Commission. The work must not be adapted and other licence terms must be abided. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nd/3.0/nz/ Copyright enquiries If you are in doubt as to whether a proposed use is covered by this licence, please contact: National Medication Safety Programme Team Health Quality & Safety Commission PO Box 25496 Wellington 6146 ACKNOWLEDGEMENTS The Health Quality & Safety Commission acknowledges the following for their assistance in producing the New Zealand Tall Man lettering list: • The Australian Commission on Safety and Quality in Health Care for advice and support in allowing its original work to be either reproduced in whole or altered in part for New Zealand as per its copyright1 • The Medication Safety and Quality Program of Clinical Excellence Commission, New South -

BC Cancer Benefit Drug List September 2021
Page 1 of 65 BC Cancer Benefit Drug List September 2021 DEFINITIONS Class I Reimbursed for active cancer or approved treatment or approved indication only. Reimbursed for approved indications only. Completion of the BC Cancer Compassionate Access Program Application (formerly Undesignated Indication Form) is necessary to Restricted Funding (R) provide the appropriate clinical information for each patient. NOTES 1. BC Cancer will reimburse, to the Communities Oncology Network hospital pharmacy, the actual acquisition cost of a Benefit Drug, up to the maximum price as determined by BC Cancer, based on the current brand and contract price. Please contact the OSCAR Hotline at 1-888-355-0355 if more information is required. 2. Not Otherwise Specified (NOS) code only applicable to Class I drugs where indicated. 3. Intrahepatic use of chemotherapy drugs is not reimbursable unless specified. 4. For queries regarding other indications not specified, please contact the BC Cancer Compassionate Access Program Office at 604.877.6000 x 6277 or [email protected] DOSAGE TUMOUR PROTOCOL DRUG APPROVED INDICATIONS CLASS NOTES FORM SITE CODES Therapy for Metastatic Castration-Sensitive Prostate Cancer using abiraterone tablet Genitourinary UGUMCSPABI* R Abiraterone and Prednisone Palliative Therapy for Metastatic Castration Resistant Prostate Cancer abiraterone tablet Genitourinary UGUPABI R Using Abiraterone and prednisone acitretin capsule Lymphoma reversal of early dysplastic and neoplastic stem changes LYNOS I first-line treatment of epidermal -

Nutritional Interventions for Autism Spectrum Disorder
Lead Article Nutritional interventions for autism spectrum disorder Downloaded from https://academic.oup.com/nutritionreviews/advance-article-abstract/doi/10.1093/nutrit/nuz092/5687289 by Florida Atlantic University user on 06 January 2020 Elisa Karhu*, Ryan Zukerman*, Rebecca S. Eshraghi, Jeenu Mittal, Richard C. Deth, Ana M. Castejon, Malav Trivedi, Rahul Mittal, and Adrien A. Eshraghi Autism spectrum disorder (ASD) is an increasingly prevalent neurodevelopmental dis- order with considerable clinical heterogeneity. With no cure for the disorder, treat- ments commonly center around speech and behavioral therapies to improve the characteristic social, behavioral, and communicative symptoms of ASD. Gastrointestinal disturbances are commonly encountered comorbidities that are thought to be not only another symptom of ASD but to also play an active role in modulating the expression of social and behavioral symptoms. Therefore, nutritional interventions are used by a majority of those with ASD both with and without clinical supervision to alleviate gastrointestinal and behavioral symptoms. Despite a consider- able interest in dietary interventions, no consensus exists regarding optimal nutritional therapy. Thus, patients and physicians are left to choose from a myriad of dietary pro- tocols. This review, summarizes the state of the current clinical and experimental liter- ature on nutritional interventions for ASD, including gluten-free and casein-free, keto- genic, and specific carbohydrate diets, as well as probiotics, polyunsaturated fatty -

Enbrace® HR DESCRIPTION: INGREDIENTS
EnBrace® HR with DeltaFolate ™ [1 NF Units] [15 mg DFE Folate] ANTI-ANEMIA PREPARATION as extrinsic/intrinsic factor concentrate plus folate. Prescription Prenatal/Vitamin Drug For Therapeutic Use Multi-phasic Capsules (30ct bottle) NDC 64661-650-30 Rx Only [DRUG] GLUTEN-FREE DESCRIPTION: EnBrace® HR is an orally administered prescription prenatal/vitamin drug for therapeutic use formulated for female macrocytic anemia patients that are in need of treatment, and are under specific direction and monitoring of vitamin B12 and vitamin B9 status by a physician. EnBrace® HR is intended for women of childbearing age who are – or desire to become, pregnant regardless of lactation status. EnBrace® HR may be prescribed for women at risk of depression as a result of folate or cobalamin deficiency - including folate-induced postpartum depression, or are at risk of folate-induced birth defects such as may be found with spina bifida and other neural tube defects (NTDs). INGREDIENTS: Cobalamin intrinsic factor complex 1 NF Units* * National Formulary Units (“NF UNITS”) equivalent to 50 mcg of active coenzyme cobalamin (as cobamamide concentrate with intrinsic factor) ALSO CONTAINS: 1 Folinic acid (B9-vitamer) 2.5 mg + 1 Control-release, citrated folic acid, DHF (B9-Provitamin) 1 mg 2 Levomefolic acid (B9 & B12- cofactor) 5.23 mg 1 6 mg DFE folate (vitamin B9) 2 9 mg DFE l-methylfolate magnesium (molar equivalent). FUNCTIONAL EXCIPIENTS: 13.6 mg FeGC as ferrous glycine cysteinate (1.5 mg 3 3,4 elemental iron ) [colorant], 25 mg ascorbates (24 mg magnesium l-ascorbate, 1 mg zinc l-ascorbate) [antioxidant], at least 23.33 mg phospholipid-omega3 complex5 [marine lipids], 500 mcg betaine (trimethylglycine) [acidifier], 1 mg magnesium l-threonate [stabilizer]. -

B-COMPLEX FORTE with VITAMIN C CAPSULES BECOSULES Capsules
For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory. B-COMPLEX FORTE WITH VITAMIN C CAPSULES BECOSULES Capsules 1. NAME OF THE MEDICINAL PRODUCT BECOSULES 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains: Thiamine Mononitrate I.P. 10 mg Riboflavin I.P. 10 mg Pyridoxine Hydrochloride I.P. 3 mg Vitamin B12 I.P. ( as STABLETS 1:100) 15 mcg Niacinamide I.P. 100 mg Calcium Pantothenate I.P. 50 mg Folic Acid I.P. 1.5 mg Biotin U.S.P. 100 mcg Ascorbic Acid I.P. (as coated) 150 mg Appropriate overages added For Therapeutic Use For a full list of excipients, see section 6.1. All strengths/presentations mentioned in this document might not be available in the market. 3. PHARMACOLOGICAL FORM Capsules 4. CLINICAL PARTICULARS 4.1 Therapeutic Indications Trademark Proprietor: Pfizer Products Inc. USA Licensed User: Pfizer Limited, India BECOSULES Capsules Page 1 of 7 LPDBCC092017 PfLEET Number: 2017-0033507 Becosules capsules are indicated in the treatment of patients with deficiencies of, or increased requirement for, vitamin B-complex, and vitamin C. Such patients and conditions include: Decreased intake because of restricted or unbalanced diet as in anorexia, diabetes mellitus, obesity and alcoholism. Reduced availability during treatment with antimicrobials which alter normal intestinal flora, in prolonged diarrhea and in chronic gastro-intestinal disorders. Increased requirements due to increased metabolic rate as in fever and tissue wasting, e.g. febrile illness, acute or chronic infections, surgery, burns and fractures. Stomatitis, glossitis, cheilosis, paraesthesias, neuralgia and dermatitis. Micronutrient deficiencies during pregnancy or lactation. -

Protocol for Look-Alike and Sound-Alike Drugs
Community Mental Health for Central Michigan PROTOCOL FOR LOOK-ALIKE AND SOUND-ALIKE DRUGS This protocol should be posted in all licensed residential group homes who contract with Community Mental Health for Central Michigan ADMINISTRATIVE GUIDELINE In an effort to improve medication safety and to meet The Joint Commission’s National Patient Safety Goal Number 3, Community Mental Health for Central Michigan (CMHCM) has identified a process to address sound-alike and look-alike drugs. The Performance Improvement Team that developed this guideline will continue to monitor new drugs on the market as they are released, review the list included with this guideline on an annual basis, and provide appropriate updates. Appropriate action to prevent errors involving the interchange of these drugs will be taken. Each CMHCM site (including direct and provider network sites) where medication is distributed or administered will post the attached lists and implement a plan for preventing drug mix-ups. This plan may consist of but not be limited to: Listing both the brand and generic names on medication records. Storing products with look-alike or sound-alike names in different locations. Employing double checks in the distribution process. Affixing “name alert” stickers to areas where look-alike or sound-alike products are stored. Changing the appearance of look-alike product names on pharmacy labels, computer screens, shelf labels and bins, and medication records by highlighting, through bold face, color, and/or tall man letters, the parts of the names that are different (e.g. hydrOXYzine, hydrALAzine). Having physicians write prescriptions using both the brand and generic names. -

Folinic Acid 2019 Newborn Use Only
Folinic acid 2019 Newborn use only Alert Folinic acid is a 5-formyl derivative of tetrahydrofolic acid. It is not the same as folic acid, but does have an equivalent vitamin activity. Also known as calcium folinate or Leucovorin. Indication Concurrent therapy with dihydrofolate reductase inhibitors such as pyrimethamine to reduce bone marrow suppression [1, 2]. Folinic acid dependent seizures and secondary causes of cerebral folate deficiency including other inborn errors of metabolism [3, 4]. Action Folinic acid is the active metabolite of folate that bypasses dihydrofolate reductase. Drug Type Metabolically active reduced form of folate (vitamin B9) Trade Name Leucovorin Presentation DBL Leucovorin Calcium tablets (calcium folinate) – 15 mg. Contains excipients including lactose monohydrate, microcrystalline cellulose, magnesium stearate. DBL Leucovorin Injection (calcium folinate) – 15 mg/2 mL, 50 mg/5 mL, 100 mg/10 mL, 300 mg/30 mL strengths available. Dosage/Interval Concurrent therapy with dihydrofolate reductase inhibitors [1, 2] 10 mg three times per week Folinic acid responsive seizures [3, 5] 2.5 mg twice a day (doses up to 8 mg/kg/day have been used) Route Oral Maximum Daily Dose Not established. Preparation/Dilution Using the injection:15-17 Measure the dose and give undiluted orally. Using the tablet: Add sterile water to 15 mg tablet to make it up to 15 mL suspension (1 mg/mL). Shake well before administration. Discard any unused liquid after administration. Administration Administer on an empty stomach (i.e. at least one hour before food or two hours after food).13 Monitoring No specific monitoring required. Contraindications Little information. -

Folate Receptor Alpha Autoantibodies in Autism Spectrum Disorders: Diagnosis, Treatment and Prevention
Journal of Personalized Medicine Review Folate Receptor Alpha Autoantibodies in Autism Spectrum Disorders: Diagnosis, Treatment and Prevention Natasha Bobrowski-Khoury 1, Vincent T. Ramaekers 2, Jeffrey M. Sequeira 3 and Edward V. Quadros 3,* 1 School of Graduate Studies, SUNY Downstate Medical Center, Brooklyn, NY 11203, USA; [email protected] 2 The Autism Center, University of Liège, 4000 Liège, Belgium; [email protected] 3 Department of Medicine, SUNY Downstate Medical Center, Brooklyn, NY 11203, USA; [email protected] * Correspondence: [email protected] Abstract: Folate deficiency and folate receptor autoimmune disorder are major contributors to infertility, pregnancy related complications and abnormal fetal development including structural and functional abnormalities of the brain. Food fortification and prenatal folic acid supplementation has reduced the incidence of neural tube defect (NTD) pregnancies but is unlikely to prevent pregnancy- related complications in the presence of folate receptor autoantibodies (FRAb). In pregnancy, these autoantibodies can block folate transport to the fetus and in young children, folate transport to the brain. These antibodies are prevalent in neural tube defect pregnancies and in developmental disorders such as cerebral folate deficiency (CFD) syndrome and autism spectrum disorder (ASD). In the latter conditions, folinic acid treatment has shown clinical improvement in some of the core ASD deficits. Early testing for folate receptor autoantibodies and intervention is likely to result Citation: Bobrowski-Khoury, N.; in a positive outcome. This review discusses the first identification of FRAb in women with a Ramaekers, V.T.; Sequeira, J.M.; history of neural tube defect pregnancy and FRAb’s association with sub-fertility and preterm birth. -

Levomefolic Acid | Medchemexpress
Inhibitors Product Data Sheet Levomefolic acid • Agonists Cat. No.: HY-14781 CAS No.: 31690-09-2 Molecular Formula: C₂₀H₂₅N₇O₆ • Molecular Weight: 459.46 Screening Libraries Target: Endogenous Metabolite; Reactive Oxygen Species Pathway: Metabolic Enzyme/Protease; Immunology/Inflammation; NF-κB Storage: Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month SOLVENT & SOLUBILITY In Vitro H2O : 5 mg/mL (10.88 mM; Need ultrasonic) DMSO : < 1 mg/mL (insoluble or slightly soluble) Mass Solvent 1 mg 5 mg 10 mg Concentration Preparing 1 mM 2.1765 mL 10.8823 mL 21.7647 mL Stock Solutions 5 mM 0.4353 mL 2.1765 mL 4.3529 mL 10 mM 0.2176 mL 1.0882 mL 2.1765 mL Please refer to the solubility information to select the appropriate solvent. BIOLOGICAL ACTIVITY Description Levomefolic acid (5-MTHF) is the natural, active form of folic acid used at the cellular level for DNA reproduction, the cysteine cycle and the regulation of homocysteine among other functions. IC50 value: Target: Folate analogLevomefolic acid has been proposed for treatment of cardiovascular disease and advanced cancers such as breast and colorectal cancers. Levomefolic acid (5-MTHF) has the prominent antioxidant activity. A high dose of 5-MTHF or folic acid does not influence Natural killer (NK) cell function in vitro. CUSTOMER VALIDATION • J Mol Med (Berl). 2019 Aug;97(8):1183-1193. See more customer validations on www.MedChemExpress.com Page 1 of 2 www.MedChemExpress.com REFERENCES [1]. Hirsch S, et al. Natural killer cell cytotoxicity is not regulated by folic acid in vitro.