L-METHYL-B6-B12 Tablets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Methionine Synthase Supports Tumor Tetrahydrofolate Pools
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.05.284521; this version posted September 7, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Methionine synthase supports tumor tetrahydrofolate pools Joshua Z. Wang1,2,#, Jonathan M. Ghergurovich1,3,#, Lifeng Yang1,2, and Joshua D. Rabinowitz1,2,* 1 Lewis-Sigler Institute for Integrative Genomics, Princeton University, Princeton, New Jersey, USA 2 Department of Chemistry, Princeton University, Princeton, New Jersey, USA 3 Department of Molecular Biology, Princeton University, Princeton, New Jersey, USA # These authors contributed equally to this work. *Corresponding author: Joshua Rabinowitz Department of Chemistry and the Lewis-Sigler Institute for Integrative Genomics, Princeton University, Washington Rd, Princeton, NJ 08544, USA Phone: (609) 258-8985; e-mail: [email protected] Conflict of Interest Disclosure: J.D.R. is a paid advisor and stockholder in Kadmon Pharmaceuticals, L.E.A.F. Pharmaceuticals, and Rafael Pharmaceuticals; a paid consultant of Pfizer; a founder, director, and stockholder of Farber Partners and Serien Therapeutics. JDR and JMG are inventors of patents in the area of folate metabolism held by Princeton University. 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.09.05.284521; this version posted September 7, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Mammalian cells require activated folates to generate nucleotides for growth and division. The most abundant circulating folate species is 5-methyl tetrahydrofolate (5-methyl- THF), which is used to synthesize methionine from homocysteine via the cobalamin-dependent enzyme methionine synthase (MTR). -

A New Mode of B Binding and the Direct Participation of A
Research Article 997 A new mode of B12 binding and the direct participation of a potassium ion in enzyme catalysis: X-ray structure of diol dehydratase Naoki Shibata1, Jun Masuda1, Takamasa Tobimatsu2, Tetsuo Toraya2*, Kyoko Suto1, Yukio Morimoto1 and Noritake Yasuoka1* Background: Diol dehydratase is an enzyme that catalyzes the adenosylcobalamin Addresses: 1Department of Life Science, Himeji (coenzyme B ) dependent conversion of 1,2-diols to the corresponding Institute of Technology, 1475-2 Kanaji, Kamigori, 12 Ako-gun, Hyogo 678-1297, Japan and 2Department aldehydes. The reaction initiated by homolytic cleavage of the cobalt–carbon bond of Bioscience and Biotechnology, Faculty of α β γ of the coenzyme proceeds by a radical mechanism. The enzyme is an 2 2 2 Engineering, Okayama University, Tsushima-Naka, heterooligomer and has an absolute requirement for a potassium ion for catalytic Okayama 700-8530, Japan. activity. The crystal structure analysis of a diol dehydratase–cyanocobalamin *Corresponding authors. complex was carried out in order to help understand the mechanism of action of E-mail: [email protected] this enzyme. [email protected] Results: The three-dimensional structure of diol dehydratase in complex with Key words: B12 enzyme, diol dehydratase, radicals, cyanocobalamin was determined at 2.2 Å resolution. The enzyme exists as a reaction mechanism, TIM barrel αβγ dimer of heterotrimers ( )2. The cobalamin molecule is bound between the Received: 16 March 1999 α and β subunits in the ‘base-on’ mode, that is, 5,6-dimethylbenzimidazole of Revisions requested: 7 April 1999 the nucleotide moiety coordinates to the cobalt atom in the lower axial position. -
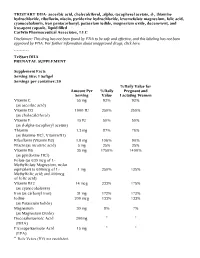
TRISTART DHA- Ascorbic Acid, Cholecalciferol, .Alpha
TRISTART DHA- ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, d-, thiamine hydrochloride, riboflavin, niacin, pyridoxine hydrochloride, levomefolate magnesium, folic acid, cyanocobalamin, iron pentacarbonyl, potassium iodide, magnesium oxide, doconexent, and icosapent capsule, liquid filled CarWin Pharmaceutical Associates, LLC Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here. ---------- TriStart DHA PRENATAL SUPPLEMENT Supplement Facts Serving Size: 1 Softgel Servings per container: 30 %Daily Value for Amount Per %Daily Pregnant and Serving Value Lactating Women Vitamin C 55 mg 92% 92% (as ascorbic acid) Vitamin D3 1000 IU 250% 250% (as cholecalciferol) Vitamin E 15 IU 50% 50% (as d-alpha-tocopheryl acetate) Thiamin 1.3 mg 87% 76% (as thiamine HCl, Vitamin B1) Riboflavin (Vitamin B2) 1.8 mg 106% 90% Niacin (as nicotinic acid) 5 mg 25% 25% Vitamin B6 35 mg 1750% 1400% (as pyridoxine HCl) Folate (as 630 mcg of L- Methylfolate Magnesium, molar equivalent to 600mcg of L- 1 mg 250% 125% Methylfolic acid; and 400mcg of folic acid) Vitamin B12 14 mcg 233% 175% (as cyanocobalamin) Iron (as carbonyl iron) 31 mg 172% 172% Iodine 200 mcg 133% 133% (as Potassium Iodide) Magnesium 30 mg 8% 7% (as Magnesium Oxide) Docosahexaenoic Acid 200mg * * (DHA) Eicosapentaenoate Acid 15 mg * * (EPA) * Daily Values (DV) not established. OTHER INGREDIENTS: Gelatin (bovine), Glycerin, Purified Water, Yellow Bees Wax, Caramel Powder, Soy Lecithin, Natural Orange Flavor, Ethyl Vanillin. Contains: Soy and Fish TriStart DHA™ Softgel capsules are dye free, lactose, gluten and sugar free. -

The Vitamin B12 Coenzyme
THE VITAMIN B12 COENZYME D. DoLPHIN, A. W. JoHNSON, R. RoDRIGO and N. SHAW Department of Chemistry, University of Nottingham, U.K. INTRODUCTION In 19•58 Barker and his associatesl-3 recognized a new coenzyme which controlled the conversion of glutamate into ß-methylaspartate by Clostridium tetanomorpkim. The coenzyme was shown4 to be related to !f;-vitamin B12, i.e. contair ing an adenine nucleotide grouping in place of the 5,6-dimethyl benziminawle nucleotide of vitamin B12, although similar coenzymes con taining btnziminazole or 5,6-dimethylbenziminazole were produced by growing C. tetanomorphum in the presence of the a ppropriate base5. Other variations of the nucleotide base have been achieved using Propionibacterium arabinosum in the presence of other purines and benziminazoles6• The pres ence of;:he coenzymes in a wide variety of micro-organisms such as several species of Actinomycetes including Streptomyces olivaceus and S. griseus has been dem( mstrated by the glutamate isomerase assay7 or by isolation. I t appears thü Vitamin B12 and its analogues are always biosynthesized in the form of their coenzymes. Preliminary physical and chemical studies sug gested that in the 5,6-dimethylbenzirninazolyl cobamide coenzyme the cyanide gr )up of vitamin B12, cyanocobalamin, was replaced by an adenine nucleoside':, 5, 8 and the determination9 of the complete structure (I; R = 5'-de·)xyadenosyl) of the coenzyme by X-ray analysis revealed the existence c f an essentially covalent bond between the cobalt atom and the S'.. carbon üom of the additional 5'-deoxyadenosine group. The molecule Me CH 2• CO· NH2 In the vitamin 8 12 coenzyme R =5' - deoxyadenosyl = Me Me 539 D. -

The Potential Protective Role of Vitamin K in Diabetic Neuropathy
VITAMINS The potential protective role of vitamin K in diabetic neuropathy DILIP MEHTA Viridis Biopharma 6/10 Jogani Industrial Complex ew cases of diabetes are symptomatic pain relief (3-5). V. N. Purav Marg, Chunabhatti increasing worldwide at a rapid Mumbai 400022, India The etiopathology of peripheral pace, with the total number of neuropathy is poorly understood and many [email protected] people with diabetes was projected factors, including dietary deficiencies, may www.viridisbiopharma.com Nto rise from 171 million in 2000 to 366 million contribute to the clinical manifestation of the in 2030 – an increase of nearly 200 million in condition. Deficiency of vitamin B12 (also only three decades. There are more cases of known as cobalamin), which results in a lack diabetes in women and urban populations, of a related compound, methylcobalamin, is with diabetes in developing countries projected manifested by megaloblastic anemia, and to double in the coming years (1). has been associated with significant Based on reports from the Centers for neurological pathology, especially peripheral Disease Control and Prevention, type 2 neuropathy (6-8). Vitamin B12 is also diabetes dult onset diabetes affects associated with the onset of diabetic approximately 9.3% of the general neuropathy. In patients with diabetic population in the United States in contrast to neuropathy, vitamin B12 deficiency may be 25.9% among those 65 years or older (2). aggravated by the use of antidiabetic agents Diabetes mellitus accounts for 90% of the such as metformin (9-11). Even short-term cases of diabetes patients (3,4). treatment with metformin causes a decrease The prevalence of type 2 diabetes in serum cobalamin, folic acid and an increases with age, higher then 25 body increase in homocysteine, which leads to mass index and the presence of the disease peripheral neuropathy in patients with in family history. -

Chemistry and Functions of Proteins
TVER STATE MEDICAL UNIVERSITY BIOCHEMISTRY DEPARTMENT CHEMISTRY AND FUNCTIONS OF PROTEINS ILLUSTRATED BIOCHEMISTRY Schemes, formulas, terms and algorithm of preparation The manual for making notes of lectures and preparation for classes Tver, 2018 AMINO ACIDS -amino acids -These are organic acids with at least a minimum of one of its hydrogen atoms in the carbon chains substituted by an amino group.( Show the radical, amino and carboxyl groups) NH2 R | C – H | COOH Proteinogenous and Nonproteinogenous Amino acids - Major proteinogenous (standard) amino acids. (Give the names of each amino acid) R R 1 H – 2 CH3 – 12 HO – CH2 – (CH3)2 CH – 3 CH3 – CH – (CH3)2 CH – CH2 – 4 13 | CH3 – CH2 – CH – OH 5 | CH 3 6 14 HS – CH2 – HOOC – CH2 – 15 CH3 – S – CH2 – CH2 – 7 HOOC – CH2 – CH2 – NH2 | – C – H - | COOH 8 16 NH2 – CO – CH2 – 9 NH2 – CO – CH2 – CH2 – 17 10 NH2 – (CH2)3 – CH2 – 18 NH2 – C – NH – (CH2)3 – CH2 – 11 || NH 19 20 СООН NH -Glycine -Arginine Alanine -Serine -Valine -Threonine Leucine -Cysteine Isoleucine -Methionine Aspartic acid -Phenylalanine Glutamic acid -Tyrosine Asparagine -Tryptophan Glutamine -Histidine Lysine -Proline •Rare proteinogenous (standard) amino acids. ( Derivatives of lysine, proline and tyrosine) NH2 | H2N – CH2 – CH – (CH2)2 – CH | | OH COOH •Nonproteinogenous amino acids. (Name and show them) NH2 NH2 | | H2N – (CH2)3 – CH HS – (CH2)2 – CH | | COOH COOH NH2 | NH2 – C – HN – (CH2)3 – CH || | O COOH Ornithine Homocysteine Citrulline 2 CLASSIFICATION OF PROTEINOGENOUS (STANDARD) AMINO ACIDS Amino acids are classified by: *The structure of the radical (show); - aliphatic amino acids -monoaminodicarboxylic amino acids -amides of amino acids -diaminomonocarboxylic amino acids -hydroxy amino acids -sulfur-containing amino acids -cyclic (aromatic and heterolytic) amino acids *the polarity of the radical (show); -Non-polar (hydrophobic –Ala, Val, Leu, Ile, Trp, Pro.) -Polar (hydrophilic). -

Chapter 7. "Coenzymes and Vitamins" Reading Assignment
Chapter 7. "Coenzymes and Vitamins" Reading Assignment: pp. 192-202, 207-208, 212-214 Problem Assignment: 3, 4, & 7 I. Introduction Many complex metabolic reactions cannot be carried out using only the chemical mechanisms available to the side-chains of the 20 standard amino acids. To perform these reactions, enzymes must rely on other chemical species known broadly as cofactors that bind to the active site and assist in the reaction mechanism. An enzyme lacking its cofactor is referred to as an apoenzyme whereas the enzyme with its cofactor is referred to as a holoenzyme. Cofactors are subdivided into essential ions and organic molecules known as coenzymes (Fig. 7.1). Essential ions, commonly metal ions, may participate in substrate binding or directly in the catalytic mechanism. Coenzymes typically act as group transfer agents, carrying electrons and chemical groups such as acyl groups, methyl groups, etc., depending on the coenzyme. Many of the coenzymes are derived from vitamins which are essential for metabolism, growth, and development. We will use this chapter to introduce all of the vitamins and coenzymes. In a few cases--NAD+, FAD, coenzyme A--the mechanisms of action will be covered. For the remainder of the water-soluble vitamins, discussion of function will be delayed until we encounter them in metabolism. We also will discuss the biochemistry of the fat-soluble vitamins here. II. Inorganic cation cofactors Many enzymes require metal cations for activity. Metal-activated enzymes require or are stimulated by cations such as K+, Ca2+, or Mg2+. Often the metal ion is not tightly bound and may even be carried into the active site attached to a substrate, as occurs in the case of kinases whose actual substrate is a magnesium-ATP complex. -

Potential Benefits of Methylcobalamin: a Review
Open Access Austin Journal of Pharmacology and Therapeutics Review Article Potential Benefits of Methylcobalamin: A Review Gupta JK* and Qureshi Shaiba Sana Department of Pharmacology, GLA University Mathura, Abstract India Methylcobalamin is an active form of vitamin B12 that helps in synthesis *Corresponding author: Jeetendra Kumar Gupta, of methionine and S-adenosylmethionine. It is required for integrity of myelin, Department of Pharmacology, Institute of Pharmaceutical neuronal function, proper red blood cell formation and DNA synthesis. The largest Research, GLA University Mathura, India group of vitamin B12 deficiency is found in typical vegetarians all over the world, which can be alleviated with its analogue Methylcobalamin. It is a beneficial Received: August 17, 2015; Accepted: September 30, drug to most of the common disorders like cardiovascular disorders, diabetes, 2015; Published: October 08, 2015 anemia, hyperhomocysteinemia and degenerative disorders. Methylcobalamin helps in the synthesis of neuronal lipids, regeneration of axonal nerves and has neuroprotective activity, which promote neurons to function in proper way and thus improves Alzheimer disease, Parkinsonism, Dementia and neuropathic syndromes. It is an approved treatment for peripheral neuropathy. Keywords: Mecobalamin; Neuropathy; Anemia; Nootropic; Dietary supplement Abbreviations essential for cell growth and replication. Sometimes the liver cannot convert cyanocobalamin into adequate amount of methylcobalamin SAMe: S-Adenosyl Methionine; ERK: Extracellular Signal- needed for proper neuronal functioning. Through enhanced Regulated Kinases; PKB: Protein Kinase B; B-globulin: Beta Globulin; methylation, it exerts its nerve cell protective effect and accelerates ENFD: Epidermal Nerve Fiber Density; DPN: Diabetic Peripheral its growth. A lot of energy is required for cyanocobalamin to remove Neuropathy; NSAIDs: Non Steroidal Anti Inflammatory Drugs; THF: its cyanide and replaces it with methyl group [3]. -

Vitamin and Mineral Requirements in Human Nutrition
P000i-00xx 3/12/05 8:54 PM Page i Vitamin and mineral requirements in human nutrition Second edition VITPR 3/12/05 16:50 Page ii WHO Library Cataloguing-in-Publication Data Joint FAO/WHO Expert Consultation on Human Vitamin and Mineral Requirements (1998 : Bangkok, Thailand). Vitamin and mineral requirements in human nutrition : report of a joint FAO/WHO expert consultation, Bangkok, Thailand, 21–30 September 1998. 1.Vitamins — standards 2.Micronutrients — standards 3.Trace elements — standards 4.Deficiency diseases — diet therapy 5.Nutritional requirements I.Title. ISBN 92 4 154612 3 (LC/NLM Classification: QU 145) © World Health Organization and Food and Agriculture Organization of the United Nations 2004 All rights reserved. Publications of the World Health Organization can be obtained from Market- ing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permis- sion to reproduce or translate WHO publications — whether for sale or for noncommercial distri- bution — should be addressed to Publications, at the above address (fax: +41 22 791 4806; e-mail: [email protected]), or to Chief, Publishing and Multimedia Service, Information Division, Food and Agriculture Organization of the United Nations, 00100 Rome, Italy. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization and the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

The Efficacy and Safety of Intramuscular Injections Of
Original Article Singapore Med J 2011; 52(12) : 868 The efficacy and safety of intramuscular injections of methylcobalamin in patients with chronic nonspecific low back pain: a randomised controlled trial Chiu C K, Low T H, Tey Y S, Singh V A, Shong H K ABSTRACT both singly or in combination with other forms Introduction:Chronic, nonspecific low back of treatment. pain is a difficult ailment to treat and poses an economic burden in terms of medical Keywords: methylcobalamin, nonspecific low expenses and productivity loss. The aim of back pain, vitamin B12 this study was to determine the efficacy and Singapore Med J 2011; 52(12): 868-873 safety of intramuscular metylcobalamin in the treatment of chronic nonspecific low back INTRODUCTION pain. Low back pain (LBP) affects a substantial proportion of the population. Almost every person will encounter an Methods: This was a double-blinded, episode of back pain at some point in one’s life. Back randomised, controlled experimental study. pain does not discriminate based on gender, age, race or 60 patients were assigned to either the culture. It disables the working adult from performing his methylcobalamin group or the placebo group. duties and paralyses the society due to the cost incurred The former received intramuscular injections in terms of treatment and productivity loss. In 1988, a of 500 mcg parenteral methylcobalamin in 1 survey was conducted in a semirural area in Malaysia. Department of ml solution three times a week for two weeks, Orthopaedic A total of 2,594 individuals from a multi-racial (Malay, Surgery, and the placebo group received 1 ml normal Chinese, Indian) community were interviewed. -

Cyanocobalamin-A Case for Withdrawal
686 Journal of the Royal Society of Medicine Volume 85 November 1992 Cyanocobalamin- a case for withdrawal: discussion paper A G Freeman MD FRCP Meadow Rise, 3 Lakeside, Swindon SN3 IQE Keywords: anaemia, pernicious; optic neuropathies; chronic cyanide intoxication; hydroxocobalamin; cyanocobalamin It seems evident that controversy still surrounds the reduced ability to detoxify the cyanide in the tobacco- treatment of pernicious anaemia and other vitamin smoke to which they are exposed'0. B12 deficiency disorders. The long quest for the 'anti- Patients with tobacco amblyopia who have normal pernicious anaemia factor' in the liver seemed to serum vitamin B12 levels need not continue therapy have ended in 1948 when pure cyanocobalamin was with intramuscular hydroxocobalamin once their isolated. This was found to be very active thera- visual acuity and visual fields have returned to peutically when given by intramuscular injection and normal providing they abstain from further smoking. was non-toxic in extremely high doses'. However, those patients who have low serum vitamin Lederle, in a recent commentary2, advocates that B12 levels or evidence of -defective vitamin B12 patients with pernicious anaemia should now be absorption will need to continue-indefinitely with treated with oral cyanocobalamin. He is not without hydroxocobalamin irrespective of their smoking support in that 40% of patients with pernicious habits as will all patients with pernicious anaemia anaemia in Sweden are being similarly treated3. and other vitamin B12 deficiency disorders who are He further states that such!- treatment is cheap at risk of developing- optic neuropathy if they and effective, produces clinical and haematological are smokers. -

Enbrace® HR DESCRIPTION: INGREDIENTS
EnBrace® HR with DeltaFolate ™ [1 NF Units] [15 mg DFE Folate] ANTI-ANEMIA PREPARATION as extrinsic/intrinsic factor concentrate plus folate. Prescription Prenatal/Vitamin Drug For Therapeutic Use Multi-phasic Capsules (30ct bottle) NDC 64661-650-30 Rx Only [DRUG] GLUTEN-FREE DESCRIPTION: EnBrace® HR is an orally administered prescription prenatal/vitamin drug for therapeutic use formulated for female macrocytic anemia patients that are in need of treatment, and are under specific direction and monitoring of vitamin B12 and vitamin B9 status by a physician. EnBrace® HR is intended for women of childbearing age who are – or desire to become, pregnant regardless of lactation status. EnBrace® HR may be prescribed for women at risk of depression as a result of folate or cobalamin deficiency - including folate-induced postpartum depression, or are at risk of folate-induced birth defects such as may be found with spina bifida and other neural tube defects (NTDs). INGREDIENTS: Cobalamin intrinsic factor complex 1 NF Units* * National Formulary Units (“NF UNITS”) equivalent to 50 mcg of active coenzyme cobalamin (as cobamamide concentrate with intrinsic factor) ALSO CONTAINS: 1 Folinic acid (B9-vitamer) 2.5 mg + 1 Control-release, citrated folic acid, DHF (B9-Provitamin) 1 mg 2 Levomefolic acid (B9 & B12- cofactor) 5.23 mg 1 6 mg DFE folate (vitamin B9) 2 9 mg DFE l-methylfolate magnesium (molar equivalent). FUNCTIONAL EXCIPIENTS: 13.6 mg FeGC as ferrous glycine cysteinate (1.5 mg 3 3,4 elemental iron ) [colorant], 25 mg ascorbates (24 mg magnesium l-ascorbate, 1 mg zinc l-ascorbate) [antioxidant], at least 23.33 mg phospholipid-omega3 complex5 [marine lipids], 500 mcg betaine (trimethylglycine) [acidifier], 1 mg magnesium l-threonate [stabilizer].