Osteogenesis Stimulators
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Orthopaedics Instructions: to Best Navigate the List, First Download This PDF File to Your Computer
Orthopaedics Instructions: To best navigate the list, first download this PDF file to your computer. Then navigate the document using the bookmarks feature in the left column. The bookmarks expand and collapse. Finally, ensure that you look at the top of each category and work down to review notes or specific instructions. Bookmarks: Bookmarks: notes or specific with expandable instructions and collapsible topics As you start using the codes, it is recommended that you also check in Index and Tabular lists to ensure there is not a code with more specificity or a different code that may be more appropriate for your patient. Copyright APTA 2016, ALL RIGHTS RESERVED. Last Updated: 09/14/16 Contact: [email protected] Orthopaedics Disorder by site: Ankle Achilles tendinopathy ** Achilles tendinopathy is not listed in ICD10 M76.6 Achilles tendinitis Achilles bursitis M76.61 Achilles tendinitis, right leg M76.62 Achilles tendinitis, left leg ** Tendinosis is not listed in ICD10 M76.89 Other specified enthesopathies of lower limb, excluding foot M76.891 Other specified enthesopathies of right lower limb, excluding foot M76.892 Other specified enthesopathies of left lower limb, excluding foot Posterior tibialis dysfunction **Posterior Tibial Tendon Dysfunction (PTTD) is not listed in ICD10 M76.82 Posterior tibial tendinitis M76.821 Posterior tibial tendinitis, right leg M76.822 Posterior tibial tendinitis, left leg M76.89 Other specified enthesopathies of lower limb, excluding foot M76.891 Other specified enthesopathies of right lower limb, -

Fractures of the Neck of the Fifth Metacarpal Bone. Medium-Term Results in 28 Cases Treated by Percutaneous Transverse Pinning I
Injury, Int. J. Care Injured 43 (2012) 242–245 Contents lists available at SciVerse ScienceDirect Injury jo urnal homepage: www.elsevier.com/locate/injury Fractures of the neck of the fifth metacarpal bone. Medium-term results in 28 cases treated by percutaneous transverse pinning V. Potenza *, R. Caterini, F. De Maio, S. Bisicchia, P. Farsetti Department of Orthopaedic Surgery, University of Rome ‘Tor Vergata’, Rome, Italy A R T I C L E I N F O A B S T R A C T Article history: The purpose of this study was to report the medium-term results in 28 patients affected by closed Accepted 27 October 2011 displaced fractures of the neck of the fifth metacarpal bone (boxer’s fracture) with an associated severe swelling of the hand, who were treated with percutaneous transverse K-wire pinning, to verify the Keywords: effectiveness of this surgical treatment. We opted for this treatment in all cases in which malrotation of Boxer’s fracture the fifth finger and volar angulation of the metacarpal head greater than 308 were associated with a Transverse pinning severe swelling of the hand. All the patients were reviewed clinically and radiologically at an average of Metacarpal neck fracture 25 months after surgery. At the final follow-up, no patient reported residual pain. All patients had full extension of the fifth finger, except two in whom we observed a limitation of the extension of the fifth metacarpophalangeal (MP) joint of about 108, without significant impairment of hand function. All patients had at least 908 flexion of the fifth MP joint and full range of motion of the interphalangeal (IP) joints. -

Functional Anatomy of the Equine Musculoskeletal System
1 CHAPTER Functional Anatomy of the Equine Musculoskeletal System ANNA DEE FAILS ANATOMIC NOMENCLATURE AND USAGE Foot Veterinary medical anatomists have been using the The foot consists of the hoof and all it encloses: the Nomina Anatomica Veterinaria, created by the Inter connective tissue corium (dermis), digital cushion, distal national Committee on Veterinary Gross Anatomical phalanx (coffin bone), most of the cartilages of the distal Nomenclature since 1968 to standardize the names of phalanx, distal interphalangeal (coffin) joint, distal part anatomical structures.46 This chapter endeavors to use the of the middle phalanx (short pastern bone), distal sesa most current, correct terms as outlined in that publication. moid (navicular) bone, podotrochlear bursa (navicular Nonetheless, equine practitioners need to be equally fluent bursa), several ligaments, tendons of insertion of the in older terminology, which is likely to be in wide usage common digital extensor and deep digital flexor mus among horse owners and equine professionals. This chap cles, blood vessels, and nerves. Skin between the heels is ter will provide useful and common synonyms for many also part of the foot. structures, along with their more technically correct terms. Figure 1.1 provides the directional terms for veteri HOOF WALL, SOLE, AND FROG nary anatomy that will be used in this chapter. With the exception of the ocular and oral cavity structures, the The hoof is continuous with the epidermis at the cor terms anterior, posterior, superior, and inferior are not onet, and the underlying corium of the hoof is likewise applicable to quadrupeds. continuous with the dermis of the skin. -

Developing Learning Models to Teach Equine Anatomy and Biomechanics
The University of Maine DigitalCommons@UMaine Honors College Spring 5-2017 Developing Learning Models to Teach Equine Anatomy and Biomechanics Zandalee E. Toothaker University of Maine Follow this and additional works at: https://digitalcommons.library.umaine.edu/honors Part of the Animal Sciences Commons, and the Veterinary Anatomy Commons Recommended Citation Toothaker, Zandalee E., "Developing Learning Models to Teach Equine Anatomy and Biomechanics" (2017). Honors College. 453. https://digitalcommons.library.umaine.edu/honors/453 This Honors Thesis is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Honors College by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. DEVELOPING LEARNING MODELS TO TEACH EQUINE ANATOMY AND BIOMECHANICS By Zandalee E. Toothaker A Thesis Submitted in Partial Fulfillment of the Requirements for a Degree with Honors (Animal and Veterinary Science) The Honors College University of Maine May 2017 Advisory Committee: Dr. Robert C. Causey, Associate Professor of Animal and Veterinary Sciences, Advisor Dr. David Gross, Adjunct Associate Professor in Honors (English) Dr. Sarah Harlan-Haughey, Assistant Professor of English and Honors Dr. Rita L. Seger, Researcher of Animal and Veterinary Sciences Dr. James Weber, Associate Professor and Animal and Veterinary Sciences © 2017 Zandalee Toothaker All Rights Reserved ABSTRACT Animal owners and professionals benefit from an understanding of an animal’s anatomy and biomechanics. This is especially true of the horse. A better understanding of the horse’s anatomy and weight bearing capabilities will allow people to treat and prevent injuries in equine athletes and work horses. -
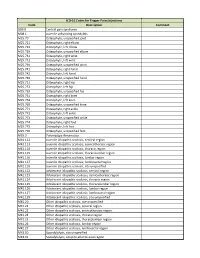
ICD-10 Codes for Trigger Point Injections
ICD-10 Codes for Trigger Point Injections Code Description Comment G89.0 Central pain syndrome M08.1 Juvenile ankylosing spondylitis M25.70 Osteophyte, unspecified joint M25.721 Osteophyte, right elbow M25.722 Osteophyte, left elbow M25.729 Osteophyte, unspecified elbow M25.731 Osteophyte, right wrist M25.732 Osteophyte, left wrist M25.739 Osteophyte, unspecified wrist M25.741 Osteophyte, right hand M25.742 Osteophyte, left hand M25.749 Osteophyte, unspecified hand M25.751 Osteophyte, right hip M25.752 Osteophyte, left hip M25.759 Osteophyte, unspecified hip M25.761 Osteophyte, right knee M25.762 Osteophyte, left knee M25.769 Osteophyte, unspecified knee M25.771 Osteophyte, right ankle M25.772 Osteophyte, left ankle M25.773 Osteophyte, unspecified ankle M25.774 Osteophyte, right foot M25.775 Osteophyte, left foot M25.776 Osteophyte, unspecified foot M35.3 Polymyalgia rheumatica M41.112 Juvenile idiopathic scoliosis, cervical region M41.113 Juvenile idiopathic scoliosis, cervicothoracic region M41.114 Juvenile idiopathic scoliosis, thoracic region M41.115 Juvenile idiopathic scoliosis, thoracolumbar region M41.116 Juvenile idiopathic scoliosis, lumbar region M41.117 Juvenile idiopathic scoliosis, lumbosacral region M41.119 Juvenile idiopathic scoliosis, site unspecified M41.122 Adolescent idiopathic scoliosis, cervical region M41.123 Adolescent idiopathic scoliosis, cervicothoracic region M41.124 Adolescent idiopathic scoliosis, thoracic region M41.125 Adolescent idiopathic scoliosis, thoracolumbar region M41.126 Adolescent idiopathic -

Metacarpal Bone Density in Carpal Tunnel Syndrome Patients Without
Original Article / Orijinal Araflt›rma 15 Metacarpal Bone Density in Carpal Tunnel Syndrome Patients Without Thenar Atrophy Tenar Atrofisi Olmayan Karpal Tünel Sendromlu Hastalarda Metakarpal Kemik Yo¤unlu¤unun De¤erlendirilmesi Serpil Savafl, Berna Okudan1, Hasan Rifat Koyuncuo¤lu2, Hakan Çelik, Tamer Karaaslan3, Mustafa Y›ld›z 4 Süleyman Demirel Üniversitesi T›p Fakültesi Fiziksel T›p ve Rehabilitasyon, 2Nöroloji, 3Beyin Cerrahisi ve 4Nükleer T›p Anabilim Dal›, Isparta, Türkiye, 1Yeditepe Üniversitesi T›p Fakültesi Nükleer T›p Anabilim Dal›, ‹stanbul, Türkiye Abstract Özet Objective: Bone loss due to thenar atrophy was reported in the me- Amaç: Premenopozal karpal tünel sendromlu (KTS) kad›nlarda te- tacarpal bones in premenopausal patients with carpal tunnel nar atrofiye ba¤l› olarak metakarpal kemik yo¤unlu¤u kayb› oldu- syndrome (CTS). The present study was designed to assess bone ¤u bilinmektedir. Bu çal›flman›n amac›, tenar atrofisi olmayan KTS’li density in the metacarpal bones in patients with CTS without hastalarda metakarpal kemik mineral yo¤unlu¤unu de¤erlendir- thenar atrophy and to correlate the metacarpal bone density with mek, metakarpal kemik yo¤unlu¤u ile elektrofizyolojik bulgular›, el the electrophysiological findings, hand strength and Boston gücü ve Boston Anketi aras›ndaki iliflkiyi belirlemektir. Questionnaire (BQ). Hastalar ve Yöntem: Çal›flmaya tenar atrofisi olmayan 30 KTS’li pre- Patients and Methods: Thirty premenopausal patients with CTS menopozal hasta ile 32 premenopozal kontrol olgu al›nd›. KTS’li without thenar atrophy were enrolled in this study. Thirty-two hastalar›n semptom fliddeti ve fonksiyonel durumlar› Bostan Anke- consecutive premenopausal women were included in the study as ti ile de¤erlendirildi. -

Case Reports Series A, B, C & D Table of Contents
ACTEC X H E • • C S A T S E R REPO Case Reports Series A, B, C & D Table of ConTents Revision Total Joint Series A, Number 1 ..................................................................................................................................................... 3 Use of opteform® to Repair failed Total Knee Prosthesis with osteolysis, Harry Schmaltz, MD Series A, Number 2 ..................................................................................................................................................... 5 Use of opteform® to Repair Acetabular osteolysis Series A, Number 4 ..................................................................................................................................................... 7 large osteolytic Defect Repair Using opteform® Through an Illiac Window, Abbott Kagan, MD Series A, Number 5 ..................................................................................................................................................... 9 Repair of Acetabular fracture and osteolysis with opteform®-Two Year follow-Up, Wayne Moody, MD fACS Series A, Number 6 ................................................................................................................................................... 11 Acetabular Reconstruction with opteform® and Reconstruction Ring, Wayne Moody, MD fACS Series A, Number 7 ................................................................................................................................................... 13 Use of opteform® -

Fracture of the Body of Hamate Associated with a Fracture of the Base of Fourth Metacarpal
CASE REPORT – OPEN ACCESS International Journal of Surgery Case Reports 4 (2013) 442–445 Contents lists available at SciVerse ScienceDirect International Journal of Surgery Case Reports j ournal homepage: www.elsevier.com/locate/ijscr Fracture of the body of hamate associated with a fracture of the base of fourth metacarpal: A case report and review of literature of the last 20 years ∗ C. Cano Gala ,D.Pescador Hernández, D.A. Rendón Díaz, J. López Olmedo, J. Blanco Blanco Department of Trauma and Orthopaedic Surgery, Virgen de la Vega Hospital, Health Center Complex of Salamanca, Paseo de San Vicente, 58, 37007 Salamanca, Spain a r t i c l e i n f o a b s t r a c t Article history: INTRODUCTION: Fractures of the carpal bones are often difficult to diagnose and treat due to the complex Received 3 December 2012 bone architecture of this region. Hamate fractures, particularly body fractures, are extremely uncommon. Received in revised form 24 January 2013 PRESENTATION OF CASE: We present a case of a coronal fracture of the hamate associated with a fracture Accepted 27 January 2013 of the base of the fourth metacarpal, which was treated by open reduction and internal fixation. Available online 13 February 2013 DISCUSSION: Some of hamate body fractures are associated with other injuries like metacarpal fractures. Its diagnosis is difficult and requires a high clinical suspicion and a proper radiological examination. This Keywords: fracture is a very rare lesion that can raise questions about their most adequate diagnostic and therapeutic Base Fourth approaches. Fracture CONCLUSION: After reviewing the literature, we conclude that there is a high rate of delay in the diagnosis Hamate of these lesions, probably due to their rarity and to the lack of radiological studies specifically targeting Metacarpal this region. -

ORIGINAL STUDY Pilot Study Evaluating a Clinical Decision Tool
Acta Orthop. Belg., 2006, 72, 411-414 ORIGINAL STUDY Pilot study evaluating a clinical decision tool on suspected scaphoid fractures Pascal STEENVOORDE, Cathrien JACOBI, Louk VA N DOORN, Jacques OSKAM From Rijnland Hospital, Leiderdorp and Leiden University Medical Center, Leiden, The Netherlands In an earlier study we have proposed a scaphoid the same time without increasing the number of decision-protocol in order to improve diagnostic missed scaphoid fractures, for if non-union of a accuracy in case of suspected scaphoid fractures. missed scaphoid occurs, outcome for the patient This pilot study evaluated this protocol. In this pilot may be very bad. This report describes the protocol study (n = 31) most cases with clinical suspicion of being evaluated in a pilot-study of 31 patients. scaphoid fractures reached a positive test result on the combined 7 clinical tests (93.5%). Using this test METHODS combination, no scaphoid fractures were missed (no false-negatives ; sensitivity 100%), but it also includ- All patients at the emergency department of our com- ed many patients with no scaphoid fracture. Many of munity Hospital, aged 16 years and older, who were sus- these, however, were found to have another fracture. pected to have a scaphoid fracture would be eligible for In total, 48% had a scaphoid fracture, 19% another the pilot study. There should be a history of an adequate fracture and 32% no fracture. In the pilot study the mechanism, like a fall on the outstretched hand. Patients proposed protocol seems to be a safe protocol, with- out missing scaphoid fractures. It leads to a reduc- tion of unnecessary plaster casting of sprained wrists and produces a marked reduction in plain radio- ■ Pascal Steenvoorde, MD, MSc, Resident in Surgery. -

Distal Third Metacarpal Bone Palmar Cortical Stress Fractures in Four Thoroughbred Racehorses C
70 EQUINE VETERINARY EDUCATION Equine vet. Educ. (2002) 14 (2) 70-76 Case Report Distal third metacarpal bone palmar cortical stress fractures in four Thoroughbred racehorses C. B. O’SULLIVAN* AND J. M. LUMSDEN Randwick Equine Centre, 3 Jane Street, Randwick, New South Wales 2031, Australia. Keywords: horse; stress/fatigue fracture; third metacarpal bone Introduction direct trainer interview and analysis of race records. The horses were at varying stages of training and none had a history of a Stress fractures of the third metacarpal bone (McIII) are a recent traumatic incident. common cause of lameness in Thoroughbred racehorses. The most commonly described location is the mid-dorsolateral Horse 1 cortex (Copelan 1979; Richardson 1984, 1999; Stashak 1987; Stover et al. 1988; Cervantes et al. 1992; Pilsworth and History Shepherd 1997; Stover 1998; Sullins 1998), but they also occur distally and proximally in the dorsal cortex (Richardson 1984, A 4-year-old gelding was presented for investigation of an 1999; Stashak 1987; Stover et al. 1988; Cervantes et al. 1992). acute onset, right forelimb lameness of grade 4/5. The gelding On the palmar aspect of McIII, stress fractures have been was undergoing a rehabilitation programme after sustaining a identified in the proximal cortex (Pleasant et al. 1992; Lloyd et right forelimb superficial digital flexor tendon injury 5 months al. 1988) and the distal condyle (Kawcak et al. 1995). previously, and commenced trotting under saddle 3 weeks Identification of stress fractures may require multiple views prior to the onset of lameness. and sequential radiographs or nuclear scintigraphy to demonstrate the lesion. -

Morphometry and Density Analysis of the Fifth Metacarpal by Amrita Unnikumaran a Thesis Submitted in Partial Fulfillment Of
Morphometry and Density Analysis of the Fifth Metacarpal by Amrita Unnikumaran A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Biomedical Engineering Department of Medical Engineering College of Engineering University of South Florida Co-Major Professor: William E. Lee III, Ph.D. Co-Major Professor: Peter Simon, Ph.D. Robert Frisina, Ph.D. Sergio Gutierrez, Ph.D. Date of Approval: October 25, 2019 Keywords: Bone Geometry, Bone Density, Computerized Tomography Scan Study, Sexual Dimorphism Copyright © 2019, Amrita Unnikumaran Dedication I dedicate this work to my family for their immense support and encouragement. Acknowledgments I would like to express my sincere gratitude to my mentor Dr. Peter Simon and Dr. William Lee for their guidance and motivation which helped me succeed. I very grateful to Dr. Robert Frisina and Dr. Sergio Gutirezz for their valuable time and suggestions which kept me motivated. I would also like to extend my gratitude to University of South Florida and Foundation for Orthopaedic Research and Education for giving me the opportunity to learn and experience high quality research. Table of Contents List of Tables ................................................................................................................................... iii List of Figures ................................................................................................................................... v Abstract ......................................................................................................................................... -

ICD-10 Codes and Definitions
ICD-10 Codes and Definitions ICD-10 Code Definition ICD-10 Code Definition COVERED DIAGNOSIS CODES FOR PROCEDURE CODES E1800, E1802 AND E1820 M12.521 TRAUMATIC ARTHROPATHY, RIGHT ELBOW M12.522 TRAUMATIC ARTHROPATHY, LEFT ELBOW M24.121 OTHER ARTICULAR CARTILAGE DISORDERS, RIGHT ELBOW M24.122 OTHER ARTICULAR CARTILAGE DISORDERS, LEFT ELBOW M24.221 DISORDER OF LIGAMENT, RIGHT ELBOW M24.222 DISORDER OF LIGAMENT, LEFT ELBOW M24.321 PATHOLOGICAL DISLOCATION OF RIGHT ELBOW, NOT ELSEWHERE CLASSIFIED M24.322 PATHOLOGICAL DISLOCATION OF LEFT ELBOW, NOT ELSEWHERE CLASSIFIED M24.421 RECURRENT DISLOCATION, RIGHT ELBOW M24.422 RECURRENT DISLOCATION, LEFT ELBOW M24.521 CONTRACTURE, RIGHT ELBOW M24.522 CONTRACTURE, LEFT ELBOW M24.821 OTHER SPECIFIC JOINT DERANGEMENTS OF RIGHT ELBOW, NOT ELSEWHERE CLASSIFIED M24.822 OTHER SPECIFIC JOINT DERANGEMENTS OF LEFT ELBOW, NOT ELSEWHERE CLASSIFIED M62.431 CONTRACTURE OF MUSCLE, RIGHT FOREARM M62.432 CONTRACTURE OF MUSCLE, LEFT FOREARM S42.401B UNSPECIFIED FRACTURE OF LOWER END OF RIGHT HUMERUS, INITIAL ENCOUNTER FOR OPEN FRACTURE S42.401D UNSPECIFIED FRACTURE OF LOWER END OF RIGHT HUMERUS, SUBSEQUENT ENCOUNTER FOR FRACTURE WITH ROUTINE HEALING S42.401G UNSPECIFIED FRACTURE OF LOWER END OF RIGHT HUMERUS, SUBSEQUENT ENCOUNTER FOR FRACTURE WITH DELAYED HEALING S42.401K UNSPECIFIED FRACTURE OF LOWER END OF RIGHT HUMERUS, SUBSEQUENT ENCOUNTER FOR FRACTURE WITH NONUNION S42.401P UNSPECIFIED FRACTURE OF LOWER END OF RIGHT HUMERUS, SUBSEQUENT ENCOUNTER FOR FRACTURE WITH MALUNION S42.402B UNSPECIFIED FRACTURE OF LOWER