Evidence for Enemy Release in Invasive Common Dace Leuciscus Leuciscus in Ireland
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Beyond Fish Edna Metabarcoding: Field Replicates Disproportionately Improve the Detection of Stream Associated Vertebrate Specie
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.26.437227; this version posted March 26, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 2 3 Beyond fish eDNA metabarcoding: Field replicates 4 disproportionately improve the detection of stream 5 associated vertebrate species 6 7 8 9 Till-Hendrik Macher1, Robin Schütz1, Jens Arle2, Arne J. Beermann1,3, Jan 10 Koschorreck2, Florian Leese1,3 11 12 13 1 University of Duisburg-Essen, Aquatic Ecosystem Research, Universitätsstr. 5, 45141 Essen, 14 Germany 15 2German Environmental Agency, Wörlitzer Platz 1, 06844 Dessau-Roßlau, Germany 16 3University of Duisburg-Essen, Centre for Water and Environmental Research (ZWU), Universitätsstr. 17 3, 45141 Essen, Germany 18 19 20 21 22 Keywords: birds, biomonitoring, bycatch, conservation, environmental DNA, mammals 23 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.03.26.437227; this version posted March 26, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 24 Abstract 25 Fast, reliable, and comprehensive biodiversity monitoring data are needed for 26 environmental decision making and management. Recent work on fish environmental 27 DNA (eDNA) metabarcoding shows that aquatic diversity can be captured fast, reliably, 28 and non-invasively at moderate costs. -

Labidesthes Sicculus
Version 2, 2015 United States Fish and Wildlife Service Lower Great Lakes Fish and Wildlife Conservation Office 1 Atherinidae Atherinidae Sand Smelt Distinguishing Features: — (Atherina boyeri) — Sand Smelt (Non-native) Old World Silversides Old World Silversides Old World (Atherina boyeri) Two widely separated dorsal fins Eye wider than Silver color snout length 39-49 lateral line scales 2 anal spines, 13-15.5 rays Rainbow Smelt (Non -Native) (Osmerus mordax) No dorsal spines Pale green dorsally Single dorsal with adipose fin Coloring: Silver Elongated, pointed snout No anal spines Size: Length: up to 145mm SL Pink/purple/blue iridescence on sides Distinguishing Features: Dorsal spines (total): 7-10 Brook Silverside (Native) 1 spine, 10-11 rays Dorsal soft rays (total): 8-16 (Labidesthes sicculus) 4 spines Anal spines: 2 Anal soft rays: 13-15.5 Eye diameter wider than snout length Habitat: Pelagic in lakes, slow or still waters Similar Species: Rainbow Smelt (Osmerus mordax), 75-80 lateral line scales Brook Silverside (Labidesthes sicculus) Elongated anal fin Images are not to scale 2 3 Centrarchidae Centrarchidae Redear Sunfish Distinguishing Features: (Lepomis microlophus) Redear Sunfish (Non-native) — — Sunfishes (Lepomis microlophus) Sunfishes Red on opercular flap No iridescent lines on cheek Long, pointed pectoral fins Bluegill (Native) Dark blotch at base (Lepomis macrochirus) of dorsal fin No red on opercular flap Coloring: Brownish-green to gray Blue-purple iridescence on cheek Bright red outer margin on opercular flap -

Beyond Fish Edna Metabarcoding: Field Replicates Disproportionately Improve the Detection of Stream Associated Vertebrate Species
Metabarcoding and Metagenomics 5: 59–71 DOI 10.3897/mbmg.5.66557 Research Article Beyond fish eDNA metabarcoding: Field replicates disproportionately improve the detection of stream associated vertebrate species Till-Hendrik Macher1, Robin Schütz1, Jens Arle2, Arne J. Beermann1,3, Jan Koschorreck2, Florian Leese1,3 1 University of Duisburg-Essen, Aquatic Ecosystem Research, Universitätsstr. 5, 45141 Essen, Germany 2 German Environment Agency, Wörlitzer Platz 1, 06844 Dessau-Roßlau, Germany 3 University of Duisburg-Essen, Centre for Water and Environmental Research (ZWU), Universitätsstr. 3, 45141 Essen, Germany Corresponding author: Till-Hendrik Macher ([email protected]) Academic editor: Pieter Boets | Received 26 March 2021 | Accepted 10 June 2021 | Published 13 July 2021 Abstract Fast, reliable, and comprehensive biodiversity monitoring data are needed for environmental decision making and management. Recent work on fish environmental DNA (eDNA) metabarcoding shows that aquatic diversity can be captured fast, reliably, and non-invasively at moderate costs. Because water in a catchment flows to the lowest point in the landscape, often a stream, it can col- lect traces of terrestrial species via surface or subsurface runoff along its way or when specimens come into direct contact with water (e.g., when drinking). Thus, fish eDNA metabarcoding data can provide information on fish but also on other vertebrate species that live in riparian habitats. This additional data may offer a much more comprehensive approach for assessing vertebrate diversity at no additional costs. Studies on how the sampling strategy affects species detection especially of stream-associated communities, however, are scarce. We therefore performed an analysis on the effects of biological replication on both fish as well as (semi-)terrestrial species detection. -

Identification of Priority Areas for the Conservation of Stream Fish Assemblages: Implications for River Management in France A
Identification of Priority Areas for the Conservation of Stream Fish Assemblages: Implications for River Management in France A. Maire, P. Laffaille, J.F. Maire, L. Buisson To cite this version: A. Maire, P. Laffaille, J.F. Maire, L. Buisson. Identification of Priority Areas for the Conservation of Stream Fish Assemblages: Implications for River Management in France. River Research and Applications, Wiley, 2016, 33 (4), pp.524-537. 10.1002/rra.3107. hal-01426354 HAL Id: hal-01426354 https://hal.archives-ouvertes.fr/hal-01426354 Submitted on 2 Jul 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License IDENTIFICATION OF PRIORITY AREAS FOR THE CONSERVATION OF STREAM FISH ASSEMBLAGES: IMPLICATIONS FOR RIVER MANAGEMENT IN FRANCE A. MAIREa*,†, P. LAFFAILLEb,c, J.-F. MAIREd AND L. BUISSONb,e a Irstea; UR HYAX, Pôle Onema-Irstea Hydroécologie des plans d’eau; Centre d’Aix-en-Provence, Aix-en-Provence, France b CNRS; UMR 5245 EcoLab, (Laboratoire Ecologie Fonctionnelle et Environnement), Toulouse, France c Université de Toulouse, INP, UPS; EcoLab; ENSAT, Castanet Tolosan, France d ONERA, The French Aerospace Lab Composites Department, Châtillon, France e Université de Toulouse, INP, UPS; EcoLab, Toulouse, France ABSTRACT Financial and human resources allocated to biodiversity conservation are often limited, making it impossible to protect all natural places, and priority areas for protection must be identified. -
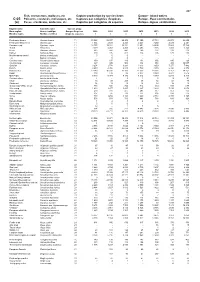
Fish, Crustaceans, Molluscs, Etc Capture Production by Species Items Europe
467 Fish, crustaceans, molluscs, etc Capture production by species items Europe - Inland waters C-05 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Europe - Eaux continentales (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Europa - Aguas continentales English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2009 2010 2011 2012 2013 2014 2015 Nombre inglés Nombre científico Grupo de especies t t t t t t t Freshwater bream Abramis brama 11 33 823 35 937 29 408 31 390 27 734 28 978 28 655 Freshwater breams nei Abramis spp 11 1 934 2 029 2 223 2 243 3 250 2 439 2 241 Common carp Cyprinus carpio 11 12 755 13 381 13 351 13 951 14 695 17 883 17 729 Tench Tinca tinca 11 3 019 4 053 2 455 2 253 1 686 1 491 1 328 Bleak Alburnus alburnus 11 682 547 441 269 231 213 219 Barbel Barbus barbus 11 300 195 412 224 205 170 188 Mediterranean barbel Barbus meridionalis 11 0 0 0 - - - - ...A Barbus cyclolepis 11 ... 0 0 - - - - Common nase Chondrostoma nasus 11 159 137 189 134 156 195 123 Crucian carp Carassius carassius 11 367 345 506 332 351 305 19 057 Goldfish Carassius auratus 11 3 254 2 778 3 293 3 613 3 653 7 277 7 482 Roach Rutilus rutilus 11 4 259 4 956 4 915 3 789 3 670 6 328 6 378 Roaches nei Rutilus spp 11 13 243 16 331 17 160 17 553 18 361 17 757 14 151 Rudd Scardinius erythrophthalmus 11 108 139 96 8 077 9 380 8 977 7 516 Orfe(=Ide) Leuciscus idus 11 5 040 5 374 5 330 4 812 5 979 6 240 5 316 Common dace Leuciscus leuciscus 11 4 .. -

And Perca Fluviatilis
International Journal of Environmental Research and Public Health Article Using Rutilus rutilus (L.) and Perca fluviatilis (L.) as Bioindicators of the Environmental Condition and Human Health: Lake Ła ´nskie,Poland Joanna Łuczy ´nska 1,* , Beata Paszczyk 1, Marek Jan Łuczy ´nski 2, Monika Kowalska-Góralska 3 , Joanna Nowosad 4 and Dariusz Kucharczyk 4 1 Chair of Commodity and Food Analysis, University of Warmia and Mazury in Olsztyn, ul. Plac Cieszy´nski1, 10-726 Olsztyn, Poland; [email protected] 2 The Stanisław Sakowicz Inland Fisheries Institute in Olsztyn, ul. Oczapowskiego 10, 10-719 Olsztyn, Poland; mj.luczynski@infish.com.pl 3 Department of Limnology and Fishery, Institute of Animal Breeding, Faculty of Biology and Animal Science, Wrocław University of Environmental and Life Sciences, ul. J. Chełmo´nskiego38 c, 51-630 Wrocław, Poland; [email protected] 4 Department of Ichthyology and Aquaculture, Warmia and Mazury University, Al. Warszawska 117A, 10-701 Olsztyn, Poland; [email protected] (J.N.); [email protected] (D.K.) * Correspondence: [email protected]; Tel.: +48-89523-4165 Received: 15 September 2020; Accepted: 16 October 2020; Published: 19 October 2020 Abstract: The aim of this study was to determine the mercury content and fatty acids profile in roach (Rutilus rutilus L.) and European perch (Perca fluviatilis L.) from Lake Ła´nskie(Poland). Mercury content was higher in the muscles than other organs in both species (p < 0.05). Mercury accumulates along the food chain of the lake’s ecosystem. The value of the bioconcentration factor (BCF) indicated that Hg had accumulated in the highest amounts in muscles and in the other organs as follows: muscles > liver > gills > gonads. -

Applied Freshwater Fish Biology an Introduction to Methods of Research and Management
How to preserve and how to exploit natural populations to be sustained for the future. Plain questions without equally plain answers Applied freshwater fish biology An introduction to methods of research and management Arne N. Linløkken, ass. professor Inland Norway University of Applied Sciences Arne N. 1 CONTENT INTRODUCTION .................................................................................................................................................. 3 Prehistory and evolution ......................................................................................................................................... 3 Short on construction and function ......................................................................................................................... 4 Morphology ........................................................................................................................................................ 4 Anatomy and physiology .................................................................................................................................... 5 European freshwater fish species ............................................................................................................................ 6 Immigration and distribution of freshwater fish in western Scandinavia ........................................................... 7 Western immigrants ........................................................................................................................ 8 -

The Living Planet Index (Lpi) for Migratory Freshwater Fish Technical Report
THE LIVING PLANET INDEX (LPI) FOR MIGRATORY FRESHWATER FISH LIVING PLANET INDEX TECHNICAL1 REPORT LIVING PLANET INDEXTECHNICAL REPORT ACKNOWLEDGEMENTS We are very grateful to a number of individuals and organisations who have worked with the LPD and/or shared their data. A full list of all partners and collaborators can be found on the LPI website. 2 INDEX TABLE OF CONTENTS Stefanie Deinet1, Kate Scott-Gatty1, Hannah Rotton1, PREFERRED CITATION 2 1 1 Deinet, S., Scott-Gatty, K., Rotton, H., Twardek, W. M., William M. Twardek , Valentina Marconi , Louise McRae , 5 GLOSSARY Lee J. Baumgartner3, Kerry Brink4, Julie E. Claussen5, Marconi, V., McRae, L., Baumgartner, L. J., Brink, K., Steven J. Cooke2, William Darwall6, Britas Klemens Claussen, J. E., Cooke, S. J., Darwall, W., Eriksson, B. K., Garcia Eriksson7, Carlos Garcia de Leaniz8, Zeb Hogan9, Joshua de Leaniz, C., Hogan, Z., Royte, J., Silva, L. G. M., Thieme, 6 SUMMARY 10 11, 12 13 M. L., Tickner, D., Waldman, J., Wanningen, H., Weyl, O. L. Royte , Luiz G. M. Silva , Michele L. Thieme , David Tickner14, John Waldman15, 16, Herman Wanningen4, Olaf F., Berkhuysen, A. (2020) The Living Planet Index (LPI) for 8 INTRODUCTION L. F. Weyl17, 18 , and Arjan Berkhuysen4 migratory freshwater fish - Technical Report. World Fish Migration Foundation, The Netherlands. 1 Indicators & Assessments Unit, Institute of Zoology, Zoological Society 11 RESULTS AND DISCUSSION of London, United Kingdom Edited by Mark van Heukelum 11 Data set 2 Fish Ecology and Conservation Physiology Laboratory, Department of Design Shapeshifter.nl Biology and Institute of Environmental Science, Carleton University, Drawings Jeroen Helmer 12 Global trend Ottawa, ON, Canada 15 Tropical and temperate zones 3 Institute for Land, Water and Society, Charles Sturt University, Albury, Photography We gratefully acknowledge all of the 17 Regions New South Wales, Australia photographers who gave us permission 20 Migration categories 4 World Fish Migration Foundation, The Netherlands to use their photographic material. -

Eurasian Dace (Leuciscus Leuciscus) Ecological Risk Screening Summary
Eurasian Dace (Leuciscus leuciscus) Ecological Risk Screening Summary U.S. Fish and Wildlife Service, May 2019 Revised, September 2019 Web Version, 12/31/2019 Photo: A. Harka. Licensed under CC BY 3.0. Available: https://commons.wikimedia.org/wiki/File:Leuciscus_leuciscus_Hungary.jpg. (May 2019). 1 Native Range and Status in the United States Native Range From Froese and Pauly (2019): “Europe and Asia: North, Baltic, White and Barents Sea basins; Caspian basin, in Volga and Ural drainages; Black Sea basin, from Danube to Dniepr drainages; Atlantic basin, in Seine drainage; Mediterranean basin from Rhône to Arch drainages (France). Only very localized in Danube main river in Romania, in Scandinavia north of 69°N and most of cenral [sic] Finland.” 1 Status in the United States This species has not been reported in the United States. This species does not appear to be in trade in the United States. Means of Introductions in the United States This species has not been reported in the United States. Remarks A previous version of this ERSS was published in 2014. From Froese and Pauly (2019): “Populations from Siberia and East Asia usually referred to Leuciscus leuciscus are distinct species, Leuciscus baicalensis and Leuciscus dzungaricus [Kottelat and Freyhof 2007].” 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing From ITIS (2019): “Kingdom Animalia Phylum Chordata Subphylum Vertebrata Superclass Osteichthyes Class Actinopterygii Subclass Neopterygii Infraclass Teleostei Superorder Ostariophysi Order Cypriniformes Superfamily Cyprinoidea Family Cyprinidae Genus Leuciscus Species Leuciscus leuciscus (Linnaeus, 1758)” From Fricke et al. (2019): “Current status: Valid as Leuciscus leuciscus (Linnaeus 1758). Leuciscidae: Leuciscinae.” Size, Weight, and Age Range From Froese and Pauly (2019): “Maturity: Lm 17.9 range ? - ? cm Max length : 40.0 cm TL male/unsexed; [Billard 1997]; common length : 15.0 cm TL male/unsexed; [Muus and Dahlström 1968]; max. -

Y4521E Cover.Ai
61 HOST-PARASITE LIST 62 CLASS CEPHALASPIDOMORPHI CLASS ACTINOPTERYGII ORDER PETRMYZONTIFORMES ORDER ANGUILLIFORMES FAMILY PETROMYZONTIDAE FAMILY ANGUILLIDAE Lampetra fluviatilis European Anguilla anguilla European eel (Linnaeus, 1758) river lamprey (Linnaeus, 1758) Zutis Status: native Upes nēģis Status: native Угорь Environment: freshwater, Речная минога Environment: freshwater, brackish, brackish, marine marine Digenea Protista Diplostomum spathaceum Ichthyophthirius multifiliis metacercaria (tanks) (Daugava River, Gulf of Riga) Trypanosoma granulosum D. petromyzifluviatilis metacercaria (Lakes Raznas, Usmas; Gulf of Riga) (Daugava River, Gulf of Riga) Myxosporea Diplostomulum sp. metacercaria Myxidium giardi (Gulf of Riga) (Lakes Liepājas, Rāznas, Usmas; Cestoda Kegums Water Reservoir; Gulf of Eubothrium sp. Riga) (Daugava River, Gulf of Riga) Digenea Proteocephalus sp. Diplostomulum sp. metacercaria (Daugava, Ogre Rivers; Gulf of (Lake Usmas) Riga) Diplostomum spathaceum Nematoda metacercaria Cucullanus truttae (Lake Rāznas, Kegums Water (Daugava River,Gulf of Riga) Reservoir, Gulf of Riga) Cystidicola farionis Sphaerostoma bramaе (Daugava River, Gulf of Riga) (Lakes Liepājas, Usmas, Gulf of Nematoda gen. sp. (Daugava River) Riga) Acanthocephala Monogenoidea Corynosoma semerme juvenile Diplozoon paradoxum (Daugava, Gauja Rivers) (Lake Liepājas) C. strumosum juvenile Pseudodactylogyrus anguillae (Daugava River) (Lake Usmas, Venta River, Gulf of Echinorhynchus gadi Riga) (Daugava River, Gulf of Riga) P. bini Hirudinida (Lake Usmas, Venta River, Gulf of Piscicola geometra (Gulf of Riga) Riga) Crustacea ?Tetraonchus sp. (Lake Liepājas) Argulus foliaceus (Gulf of Riga) Cestoda Remarks: The river lamprey is anadromous Bothriocephalus claviceps species. Adults occur in the Baltic Sea and (Lakes Rāznas, Rušons, Usmas; Gulf of Riga, entering rivers for spawning. It Kegums Water Reservoir, Venta is a commercially important species with an River; Gulf of Riga) annual catch of 70–170 tonnes (Plikšs & Proteocephalus macrocephalus Aleksejevs 1998). -

Glossary of Japanese Fisheries Tirms 1
states Department of the Interior, J. A. Krug, Secretary Fish and Wildlife Service, Albert M. Day, Director -... -------- Fishery Leaflet 220 Chica 0 54 Ill. 17126 March 194'1 GLOSSARY OF JAPANESE FISHERIES TERMS General Headquarters Supreme Commander for the Allied Powers Natural Resources Section Report No. 63 Tokyo 1946 GLOSSARY OF J.APAllISE FISHlWES ~S 1./ 1. Flsh and fishing play such an important role in Japanese life tbat an extensive and complicated fisheries vocabulary bas evolved. Bach of the hundreds of kinds of fish, shellfish, and seaweed bas several vernacular names, the wide alsortment of prepared seafood adds many ~ore words; and the variety of fishing gear bas a large specialized nomen clatllre. 2. An interpreter or translator who 1s not trained infisberias terminology finds himself contused by the many Japanese terms, some of which have no exact cOllnterpart in Engl1ah. The Japanese when translating their own phraseology into English make freqll8nt unintentional mistakes because of the complicated nature of the subject matter. Many organisms which are abllndant in Japan ~re not to be found in any part of the Englis~speaking world. and attempts at t~nslatio~ are often inacc~Ge. 3. This glossary was prepared to establish a standardized vocabulary It should help the members of ~e Occupation Forces to understand Japanese publications and reports. It should help the Jap~ese atlthor1ties in translating their reports into English. 4. The best accepted common names for commercially important marlne_ and freshwate;r animals and plants are presented. Countless local vernacu lar names for fish. shellfish. and seaweed are 1n use in various parts of Japan. -

Use of the Chromosomal Co-Location of the Minor 5S and the Major 28S Rdna As a Cytogenetic Marker Within the Genus Leuciscus (Pisces, Cyprinidae)
Folia biologica (Kraków), vol. 58 (2010), No 3-4 doi:10.3409/fb58_3-4.245-249 UseoftheChromosomalCo-locationoftheMinor5SandtheMajor28S rDNAasaCytogeneticMarkerwithinthe Genus Leuciscus (Pisces, Cyprinidae)* LechKIRTIKLIS,KatarzynaPORYCKA,AlicjaBOROÑ,Jean-PierreCOUTANCEAU, andAgnesDETTAI Accepted May 25, 2010 KIRTIKLIS L., PORYCKA K., BOROÑ A., COUTANCEAU J-P., DETTAI A. 2010. Use of the chromosomal co-location of the minor 5S and the major 28S rDNA as a cytogenetic marker within the genus Leuciscus (Pisces, Cyprinidae). Folia biol. (Kraków) 58: 245-249. The chromosomes of three species from the genus Leuciscus (the ide L. idus, the European chub L. cephalus and the common dace L. leuciscus) were examined with the FISH technique for5Sand28SrDNAprobes.Theanalysisshowedthatamongthe three examined species, 5S rDNA signals were located on two large and four small subtelocentric chromosomes in L. leuciscus, on one large and five small subtelocentric chromosomes in L. idus, while in L. cephalus the probe signals were found on two metacentric chromosomes and one large and one small subtelocentric chromosome pairs. In all analysed species, the 28S rDNA probe signalswereplacedononlyonechromosomepair,subtelocentricinthecommondaceandthe European chub, and submetacentric in the ide. The three species differed in the number of sites in which both probe signals were present. In conclusion, the co-location of the 5S and 28SrDNAprovedtobeausefulcytogeneticmarkeramongthestudied fishes. Moreover, this marker could be adapted to other cyprinids. Key words: Chromosome, FISH, rDNA, Leuciscus, gene mapping, species identification. Lech KIRTIKLIS,KatarzynaPORYCKA, Alicja BOROÑ,DepartmentofZoology,FacultyofBiology, University of Warmia and Mazury in Olsztyn, Oczapowskiego 5, 10-718 Olsztyn, Poland. E-mail: [email protected] [email protected] [email protected] Jean-Pierre COUTANCEAU, Agnes DETTAI, Département Systématique et Evolution, Museum National dHistoire Naturelle, 43, rue Cuvier, 75005 Paris, France.