Eurasian Dace (Leuciscus Leuciscus) Ecological Risk Screening Summary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CAT Vertebradosgt CDC CECON USAC 2019
Catálogo de Autoridades Taxonómicas de vertebrados de Guatemala CDC-CECON-USAC 2019 Centro de Datos para la Conservación (CDC) Centro de Estudios Conservacionistas (Cecon) Facultad de Ciencias Químicas y Farmacia Universidad de San Carlos de Guatemala Este documento fue elaborado por el Centro de Datos para la Conservación (CDC) del Centro de Estudios Conservacionistas (Cecon) de la Facultad de Ciencias Químicas y Farmacia de la Universidad de San Carlos de Guatemala. Guatemala, 2019 Textos y edición: Manolo J. García. Zoólogo CDC Primera edición, 2019 Centro de Estudios Conservacionistas (Cecon) de la Facultad de Ciencias Químicas y Farmacia de la Universidad de San Carlos de Guatemala ISBN: 978-9929-570-19-1 Cita sugerida: Centro de Estudios Conservacionistas [Cecon]. (2019). Catálogo de autoridades taxonómicas de vertebrados de Guatemala (Documento técnico). Guatemala: Centro de Datos para la Conservación [CDC], Centro de Estudios Conservacionistas [Cecon], Facultad de Ciencias Químicas y Farmacia, Universidad de San Carlos de Guatemala [Usac]. Índice 1. Presentación ............................................................................................ 4 2. Directrices generales para uso del CAT .............................................. 5 2.1 El grupo objetivo ..................................................................... 5 2.2 Categorías taxonómicas ......................................................... 5 2.3 Nombre de autoridades .......................................................... 5 2.4 Estatus taxonómico -

Review and Meta-Analysis of the Environmental Biology and Potential Invasiveness of a Poorly-Studied Cyprinid, the Ide Leuciscus Idus
REVIEWS IN FISHERIES SCIENCE & AQUACULTURE https://doi.org/10.1080/23308249.2020.1822280 REVIEW Review and Meta-Analysis of the Environmental Biology and Potential Invasiveness of a Poorly-Studied Cyprinid, the Ide Leuciscus idus Mehis Rohtlaa,b, Lorenzo Vilizzic, Vladimır Kovacd, David Almeidae, Bernice Brewsterf, J. Robert Brittong, Łukasz Głowackic, Michael J. Godardh,i, Ruth Kirkf, Sarah Nienhuisj, Karin H. Olssonh,k, Jan Simonsenl, Michał E. Skora m, Saulius Stakenas_ n, Ali Serhan Tarkanc,o, Nildeniz Topo, Hugo Verreyckenp, Grzegorz ZieRbac, and Gordon H. Coppc,h,q aEstonian Marine Institute, University of Tartu, Tartu, Estonia; bInstitute of Marine Research, Austevoll Research Station, Storebø, Norway; cDepartment of Ecology and Vertebrate Zoology, Faculty of Biology and Environmental Protection, University of Lodz, Łod z, Poland; dDepartment of Ecology, Faculty of Natural Sciences, Comenius University, Bratislava, Slovakia; eDepartment of Basic Medical Sciences, USP-CEU University, Madrid, Spain; fMolecular Parasitology Laboratory, School of Life Sciences, Pharmacy and Chemistry, Kingston University, Kingston-upon-Thames, Surrey, UK; gDepartment of Life and Environmental Sciences, Bournemouth University, Dorset, UK; hCentre for Environment, Fisheries & Aquaculture Science, Lowestoft, Suffolk, UK; iAECOM, Kitchener, Ontario, Canada; jOntario Ministry of Natural Resources and Forestry, Peterborough, Ontario, Canada; kDepartment of Zoology, Tel Aviv University and Inter-University Institute for Marine Sciences in Eilat, Tel Aviv, -

Beyond Fish Edna Metabarcoding: Field Replicates Disproportionately Improve the Detection of Stream Associated Vertebrate Specie
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.26.437227; this version posted March 26, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 2 3 Beyond fish eDNA metabarcoding: Field replicates 4 disproportionately improve the detection of stream 5 associated vertebrate species 6 7 8 9 Till-Hendrik Macher1, Robin Schütz1, Jens Arle2, Arne J. Beermann1,3, Jan 10 Koschorreck2, Florian Leese1,3 11 12 13 1 University of Duisburg-Essen, Aquatic Ecosystem Research, Universitätsstr. 5, 45141 Essen, 14 Germany 15 2German Environmental Agency, Wörlitzer Platz 1, 06844 Dessau-Roßlau, Germany 16 3University of Duisburg-Essen, Centre for Water and Environmental Research (ZWU), Universitätsstr. 17 3, 45141 Essen, Germany 18 19 20 21 22 Keywords: birds, biomonitoring, bycatch, conservation, environmental DNA, mammals 23 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.03.26.437227; this version posted March 26, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 24 Abstract 25 Fast, reliable, and comprehensive biodiversity monitoring data are needed for 26 environmental decision making and management. Recent work on fish environmental 27 DNA (eDNA) metabarcoding shows that aquatic diversity can be captured fast, reliably, 28 and non-invasively at moderate costs. -

Labidesthes Sicculus
Version 2, 2015 United States Fish and Wildlife Service Lower Great Lakes Fish and Wildlife Conservation Office 1 Atherinidae Atherinidae Sand Smelt Distinguishing Features: — (Atherina boyeri) — Sand Smelt (Non-native) Old World Silversides Old World Silversides Old World (Atherina boyeri) Two widely separated dorsal fins Eye wider than Silver color snout length 39-49 lateral line scales 2 anal spines, 13-15.5 rays Rainbow Smelt (Non -Native) (Osmerus mordax) No dorsal spines Pale green dorsally Single dorsal with adipose fin Coloring: Silver Elongated, pointed snout No anal spines Size: Length: up to 145mm SL Pink/purple/blue iridescence on sides Distinguishing Features: Dorsal spines (total): 7-10 Brook Silverside (Native) 1 spine, 10-11 rays Dorsal soft rays (total): 8-16 (Labidesthes sicculus) 4 spines Anal spines: 2 Anal soft rays: 13-15.5 Eye diameter wider than snout length Habitat: Pelagic in lakes, slow or still waters Similar Species: Rainbow Smelt (Osmerus mordax), 75-80 lateral line scales Brook Silverside (Labidesthes sicculus) Elongated anal fin Images are not to scale 2 3 Centrarchidae Centrarchidae Redear Sunfish Distinguishing Features: (Lepomis microlophus) Redear Sunfish (Non-native) — — Sunfishes (Lepomis microlophus) Sunfishes Red on opercular flap No iridescent lines on cheek Long, pointed pectoral fins Bluegill (Native) Dark blotch at base (Lepomis macrochirus) of dorsal fin No red on opercular flap Coloring: Brownish-green to gray Blue-purple iridescence on cheek Bright red outer margin on opercular flap -

Rough Fish”: Paradigm Shift in the Conservation of Native Fishes Andrew L
PERSPECTIVE Goodbye to “Rough Fish”: Paradigm Shift in the Conservation of Native Fishes Andrew L. Rypel | University of California, Davis, Department of Wildlife, Fish and Conservation Biology, 1 Shields Ave, Davis, CA 95616 | University of California, Davis, Center for Watershed Sciences, Davis, CA. E-mail: [email protected] Parsa Saffarinia | University of California, Davis, Department of Wildlife, Fish and Conservation Biology, Davis, CA Caryn C. Vaughn | University of Oklahoma, Oklahoma Biological Survey and Department of Biology, Norman, OK Larry Nesper | University of Wisconsin–Madison, Department of Anthropology, Madison, WI Katherine O’Reilly | University of Notre Dame, Department of Biological Sciences, Notre Dame, IN Christine A. Parisek | University of California, Davis, Center for Watershed Sciences, Davis, CA | University of California, Davis, Department of Wildlife, Fish and Conservation Biology, Davis, CA | The Nature Conservancy, Science Communications, Boise, ID Peter B. Moyle | University of California, Davis, Center for Watershed Sciences, Davis, CA Nann A. Fangue | University of California, Davis, Department of Wildlife, Fish and Conservation Biology, Davis, CA Miranda Bell- Tilcock | University of California, Davis, Center for Watershed Sciences, Davis, CA David Ayers | University of California, Davis, Center for Watershed Sciences, Davis, CA | University of California, Davis, Department of Wildlife, Fish and Conservation Biology, Davis, CA Solomon R. David | Nicholls State University, Department of Biological Sciences, Thibodaux, LA While sometimes difficult to admit, perspectives of European and white males have overwhelmingly dominated fisheries science and management in the USA. This dynamic is exemplified by bias against “rough fish”— a pejorative ascribing low- to- zero value for countless native fishes. One product of this bias is that biologists have ironically worked against conservation of diverse fishes for over a century, and these problems persist today. -

Effectiveness of FISK, an Invasiveness Screening Tool for Nonnative
Risk Analysis DOI: 10.1111/risa.12050 Effectiveness of FISK, an Invasiveness Screening Tool for Non-Native Freshwater Fishes, to Perform Risk Identification Assessments in the Iberian Peninsula David Almeida,1,2 Filipe Ribeiro,3,4 Pedro M. Leunda,5,6 Lorenzo Vilizzi,7 and Gordon H. Copp1,2,6,8,∗ Risk assessments are crucial for identifying and mitigating impacts from biological invasions. The Fish Invasiveness Scoring Kit (FISK) is a risk identification (screening) tool for fresh- water fishes consisting of two subject areas: biogeography/history and biology/ecology. Ac- cording to the outcomes, species can be classified under particular risk categories. The aim of this study was to apply FISK to the Iberian Peninsula, a Mediterranean climate region highly important for freshwater fish conservation due to a high level of endemism. In total, 89 fish species were assessed by three independent assessors. Results from receiver operating characteristic analysis showed that FISK can discriminate reliably between noninvasive and invasive fishes for Iberia, with a threshold of 20.25, similar to those obtained in several regions around the world. Based on mean scores, no species was categorized as “low risk,” 50 species as “medium risk,” 17 as “moderately high risk,” 11 as “high risk,” and 11 as “very high risk.” The highest scoring species was goldfish Carassius auratus. Mean certainty in response was above the category “mostly certain,” ranging from tinfoil barb Barbonymus schwanenfeldii with the lowest certainty to eastern mosquitofish Gambusia holbrooki with the highest level. Pair-wise comparison showed significant differences between one assessor and the other two on mean certainty, with these two assessors showing a high coincidence rate for the species categorization. -

NAME of SPECIES: Ide (Leuciscus Idus)
NAME OF SPECIES: Ide (Leuciscus idus) A. CURRENT STATUS AND DISTRIBUTION 1. In Wisconsin? a. YES NO b. Abundance: c. Geographic Range: d. Type of Waters Invaded (rivers, ponds, lakes, etc): (in other states) pools in rivers and slow-flowing or still water, ponds, lakes, estuaries e. Historical Status and Rate of Spread in Wisconsin: 2. Invasive in Similar Climate YES NO Zones Where: Maine, northeastern US, Pennsylvania (localized populations in these states) 3. Similar Habitat Invaded YES NO Elsewhere Where: 4. In Surrounding States YES NO Where: Reports of introductions from MN, IL, IN, OH, though it's unclear if any reproducing populations remain 5. Competitive Ability High: Tolerate a wide range of conditions Low: Reports of localized reproducing populations in a few locations, but unclear if any still exist. Poor records on this fish, so its true extent in the US is not clear. However, it's been here since the 1800s and has failed to thrive outside of a few small ponds, etc. B. ESTABLISHMENT POTENTIAL AND LIFE HISTORY TRAITS 1. Temperature: Range: 4 deg. C - 20 deg. C Upper lethal temps. for larvae and juveniles aclimated to 6 - 22 deg. C was 24 - 29 deg. C (was lower for fish aclimated to lower temps) 2. Spawning Temperature: Range: Begins at water temps from 5 - 14 deg. C, optimal temp. for egg development 12 - 18 deg. C 3. Number of Eggs: Range: large range reported throughout Europe, from 15,000 - 263,000 egg per female 4. Preferred Spawning weedy, shallow areas where adhesive eggs attach to stones or Substrate: vegetation 5. -

Beyond Fish Edna Metabarcoding: Field Replicates Disproportionately Improve the Detection of Stream Associated Vertebrate Species
Metabarcoding and Metagenomics 5: 59–71 DOI 10.3897/mbmg.5.66557 Research Article Beyond fish eDNA metabarcoding: Field replicates disproportionately improve the detection of stream associated vertebrate species Till-Hendrik Macher1, Robin Schütz1, Jens Arle2, Arne J. Beermann1,3, Jan Koschorreck2, Florian Leese1,3 1 University of Duisburg-Essen, Aquatic Ecosystem Research, Universitätsstr. 5, 45141 Essen, Germany 2 German Environment Agency, Wörlitzer Platz 1, 06844 Dessau-Roßlau, Germany 3 University of Duisburg-Essen, Centre for Water and Environmental Research (ZWU), Universitätsstr. 3, 45141 Essen, Germany Corresponding author: Till-Hendrik Macher ([email protected]) Academic editor: Pieter Boets | Received 26 March 2021 | Accepted 10 June 2021 | Published 13 July 2021 Abstract Fast, reliable, and comprehensive biodiversity monitoring data are needed for environmental decision making and management. Recent work on fish environmental DNA (eDNA) metabarcoding shows that aquatic diversity can be captured fast, reliably, and non-invasively at moderate costs. Because water in a catchment flows to the lowest point in the landscape, often a stream, it can col- lect traces of terrestrial species via surface or subsurface runoff along its way or when specimens come into direct contact with water (e.g., when drinking). Thus, fish eDNA metabarcoding data can provide information on fish but also on other vertebrate species that live in riparian habitats. This additional data may offer a much more comprehensive approach for assessing vertebrate diversity at no additional costs. Studies on how the sampling strategy affects species detection especially of stream-associated communities, however, are scarce. We therefore performed an analysis on the effects of biological replication on both fish as well as (semi-)terrestrial species detection. -

Identification of Priority Areas for the Conservation of Stream Fish Assemblages: Implications for River Management in France A
Identification of Priority Areas for the Conservation of Stream Fish Assemblages: Implications for River Management in France A. Maire, P. Laffaille, J.F. Maire, L. Buisson To cite this version: A. Maire, P. Laffaille, J.F. Maire, L. Buisson. Identification of Priority Areas for the Conservation of Stream Fish Assemblages: Implications for River Management in France. River Research and Applications, Wiley, 2016, 33 (4), pp.524-537. 10.1002/rra.3107. hal-01426354 HAL Id: hal-01426354 https://hal.archives-ouvertes.fr/hal-01426354 Submitted on 2 Jul 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License IDENTIFICATION OF PRIORITY AREAS FOR THE CONSERVATION OF STREAM FISH ASSEMBLAGES: IMPLICATIONS FOR RIVER MANAGEMENT IN FRANCE A. MAIREa*,†, P. LAFFAILLEb,c, J.-F. MAIREd AND L. BUISSONb,e a Irstea; UR HYAX, Pôle Onema-Irstea Hydroécologie des plans d’eau; Centre d’Aix-en-Provence, Aix-en-Provence, France b CNRS; UMR 5245 EcoLab, (Laboratoire Ecologie Fonctionnelle et Environnement), Toulouse, France c Université de Toulouse, INP, UPS; EcoLab; ENSAT, Castanet Tolosan, France d ONERA, The French Aerospace Lab Composites Department, Châtillon, France e Université de Toulouse, INP, UPS; EcoLab, Toulouse, France ABSTRACT Financial and human resources allocated to biodiversity conservation are often limited, making it impossible to protect all natural places, and priority areas for protection must be identified. -

Telestes Turskyi Brought to You by CORE
Croatian Journal of Fisheries, 2014, 72, 123 – 124 View metadata,T. Mihinjač citation et and al.: similar"reatened papers $shes at of core.ac.uk the world: Telestes turskyi brought to you by CORE http://dx.doi.org/10.14798/72.3.749 CODEN RIBAEG ISSN 1330-061X THREATENED FISHES OF THE WORLD: Telestes turskyi (Heckel, 1843) (Cyprinidae) Tanja Mihinjač 1, Zoran Marčić 1, Milorad Mrakovčić 1, Perica Mustafić 1, Davor Zanella 1, Marko Ćaleta*2 1 University of Zagreb, Faculty of Science, Department of Zoology, Rooseveltov trg 6, 10000 Zagreb, Croatia 2 University of Zagreb, Faculty of Teacher Education, Savska cesta 77, 10000 Zagreb, Croatia * Corresponding author, E-mail: [email protected] ARTICLE INFO ABSTRACT Received: 1 June 2014 Tursky dace, Telestes turskyi , is a freshwater species endemic to the Received in revised form: 26 June 2014 Adriatic drainage. This species is found only in two rivers in Croatia, Accepted: 5 July 2014 the River Krka and River Čikola, and is protected by Croatian law. Major Avaible online: 27 August 2014 threats for this species are extremely limited distribution, river regulation, water extraction and pollution. Suggested conservation actions for this species are: habitat protection, bans on regulation and alteration Keywords: of watercourse and restriction of introduction of alien fish species. Telestes turskyi Cyprinidae Adriatic watershed Conservation SYNONYM Leuciscus turskyi (Heckel, 1843); Squalius turskyi Heckel, 1843 COMMON NAMES Turski klen (Cro); Tursky dace, Čikola riffle dace (Eng) CONSERVATION STATUS Fig 1. Telestes turskyi 180 mm TL (photo by Perica Mustafić, May 2011) IUCN Red list: critically endangered (Crivelli, 2006) Croatia: critically endangered (Mrakovčić et al., 2006) DISTRIBUTION IDENTIFICATION Endemic to the Adriatic drainage. -
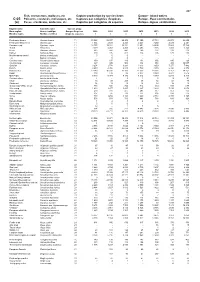
Fish, Crustaceans, Molluscs, Etc Capture Production by Species Items Europe
467 Fish, crustaceans, molluscs, etc Capture production by species items Europe - Inland waters C-05 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Europe - Eaux continentales (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Europa - Aguas continentales English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2009 2010 2011 2012 2013 2014 2015 Nombre inglés Nombre científico Grupo de especies t t t t t t t Freshwater bream Abramis brama 11 33 823 35 937 29 408 31 390 27 734 28 978 28 655 Freshwater breams nei Abramis spp 11 1 934 2 029 2 223 2 243 3 250 2 439 2 241 Common carp Cyprinus carpio 11 12 755 13 381 13 351 13 951 14 695 17 883 17 729 Tench Tinca tinca 11 3 019 4 053 2 455 2 253 1 686 1 491 1 328 Bleak Alburnus alburnus 11 682 547 441 269 231 213 219 Barbel Barbus barbus 11 300 195 412 224 205 170 188 Mediterranean barbel Barbus meridionalis 11 0 0 0 - - - - ...A Barbus cyclolepis 11 ... 0 0 - - - - Common nase Chondrostoma nasus 11 159 137 189 134 156 195 123 Crucian carp Carassius carassius 11 367 345 506 332 351 305 19 057 Goldfish Carassius auratus 11 3 254 2 778 3 293 3 613 3 653 7 277 7 482 Roach Rutilus rutilus 11 4 259 4 956 4 915 3 789 3 670 6 328 6 378 Roaches nei Rutilus spp 11 13 243 16 331 17 160 17 553 18 361 17 757 14 151 Rudd Scardinius erythrophthalmus 11 108 139 96 8 077 9 380 8 977 7 516 Orfe(=Ide) Leuciscus idus 11 5 040 5 374 5 330 4 812 5 979 6 240 5 316 Common dace Leuciscus leuciscus 11 4 .. -

Karyotype of Persian Chub, Petroleuciscus Persidis (Coad, 1981) (Actinopterygii: Cyprinidae) from Southern Iran
TurkJZool 30(2006)137-139 ©TÜB‹TAK KaryotypeofPersianChub,Petroleuciscuspersidis (Coad,1981) (Actinopterygii:Cyprinidae)fromSouthernIran H.R.ESMAEILI*,Z.PIRAVAR DepartmentofBiology,CollegeofSciences,ShirazUniversity,Shiraz,71454-IRAN Received:25.04.2005 Abstract: ThediploidchromosomenumberofPersianchub, Petroleuciscuspersidis (Coad,1981),was2n=50,comprising29 metacentric,18submetacentric,and3subtelocentricchromosomesandthenumberofarmswas97.Adetailedkaryotypeofthis endemiccyprinidfishofsouthernIranwasestablishedforthefirsttimeinthisstudy. KeyWords: Cyprinidkaryology,Petroleuciscuspersidis,Iran Introduction attentioninrecentyears(Ozouf-CostazandForesti, Thecarp,orminnowfamily(Cyprinidae),isoneofthe 1992;Galettietal.,2000).Fishchromosomedatahave mostwidespreadandspeciosefamiliesoffishinthe greatimportanceinstudiesconcerningevolutionary world;certainlythemostspecioseinfreshwaterand systematics,aquaculture,andmutagenesis(Al-Sabti, possiblythelargestfamilyofvertebrates(Coad,2005). 1991).Theincreasingimportanceofchromosomal ThisfamilyisfoundinNorthAmerica,Eurasia,andAfrica. studiesoffishandthelackofdataonIranianfish Thereareover2100species,almost10%oftheworld’s karyotypespromptedustodoakaryotypestudyof fish(Coad,2005).InIran,thisfamilyisrepresentedby Petroleuciscuspersidis .Tothebestofourknowledge, speciesfoundinallthemajordrainagebasins.It thisisthefirstreportofitskind.Hence,theprimaryaim comprisesabout50%oftheIranianfreshwaterfish ofthisstudywastodescribethechromosomesand fauna(Coad,1995)andthereforecyprinidfishrepresent karyotypeofPetroleuciscuspersidis