Applied Freshwater Fish Biology an Introduction to Methods of Research and Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cottus Poecilopus Heckel, 1836, in the River Javorin- Ka, the Tatra
Oecologia Montana 2018, Cottus poecilopus Heckel, 1836, in the river Javorin- 27, 21-26 ka, the Tatra mountains, Slovakia M. JANIGA, Jr. In Tatranská Javorina under Muráň mountain, a small fish nursery was built by Christian Kraft von Institute of High Mountain Biology University of Hohenlohe around 1930. The most comprehensive Žilina, Tatranská Javorina 7, SK-059 56, Slovakia; studies on fish from the Tatra mountains were writ- e-mail:: [email protected] ten by professor Václav Dyk (1957; 1961), Dyk and Dyková (1964a,b; 1965), who studied altitudinal distribution of fish, describing the highest points where fish were found. His studies on fish were likely the most complex studies of their kind during that period. Along with his wife Sylvia, who illus- Abstract. This study focuses on the Cottus poe- trated his studies, they published the first realistic cilopus from the river Javorinka in the north-east studies on fish from the Tatra mountains including High Tatra mountains, Slovakia. The movement the river Javorinka (Dyk and Dyková 1964a). Feri- and residence of 75 Alpine bullhead in the river anc (1948) published the first Slovakian nomenclature were monitored and carefully recorded using GPS of fish in 1948. Eugen K. Balon (1964; 1966) was the coordinates. A map representing their location in next famous ichthyologist who became a recognised the river was generated. This data was collected in expert in the fish fauna of the streams of the Tatra the spring and summer of 2016 and in the autumn mountains, the river Poprad, and various high moun- of 2017. Body length and body weight of 67 Alpine tain lakes. -

Trophic Relationships in Dutch Reservoirs Recently Invaded by Ponto-Caspian Species: Insights from Fish Trends and Stable Isotope Analysis
Aquatic Invasions (2019) Volume 14, Issue 2: 280–298 CORRECTED PROOF Research Article Trophic relationships in Dutch reservoirs recently invaded by Ponto-Caspian species: insights from fish trends and stable isotope analysis Yvon J.M. Verstijnen1,*, Esther C.H.E.T. Lucassen1,2, Marinus van der Gaag3, Arco J. Wagenvoort5, Henk Castelijns4, Henk A.M. Ketelaars4, Gerard van der Velde3,6,7 and Alfons J.P. Smolders1,2 1B-WARE Research Centre, Radboud University, Toernooiveld 1, 6525 ED Nijmegen, The Netherlands 2Department of Aquatic Ecology and Environmental Biology, Institute for Water and Wetland Research, Radboud University, Heyendaalseweg 135, 6525 AJ Nijmegen, The Netherlands 3Department of Animal Ecology and Physiology, Institute for Water and Wetland Research, Radboud University, Heyendaalseweg 135, 6525 AJ Nijmegen, The Netherlands 4Evides Water Company, PO Box 4472, 3006 AL Rotterdam, The Netherlands 5AqWa, Voorstad 45, 4461 RT Goes, The Netherlands 6Naturalis Biodiversity Center, P.O. 9517, 2300 RA Leiden, The Netherlands 7Netherlands Centre of Expertise on Exotic Species (NEC-E). Heyendaalseweg 135, 6525 AJ Nijmegen, The Netherlands Author e-mails: [email protected] (YJMV), [email protected] (ECHETL), [email protected] (MG), [email protected], (AJW), [email protected] (HC), [email protected] (HAMK), [email protected] (VG), [email protected] (AJPS) *Corresponding author Citation: Verstijnen YJM, Lucassen ECHET, van der Gaag M, Wagenvoort AJ, Abstract Castelijns H, Ketelaars HAM, van der Velde G, Smolders AJP (2019) Trophic Invasive species can directly or indirectly alter (a)biotic characteristics of ecosystems, relationships in Dutch reservoirs recently resulting in changing energy flows through the food web. -

Trout Stocking in SAC Rivers. Phase 1: Review of Stocking Practice
Trout stocking in SAC rivers. Phase 1: Review of stocking practice Science Report: SC030211/SR1 SCHO0707BMZC-E-P The Environment Agency is the leading public body protecting and improving the environment in England and Wales. It’s our job to make sure that air, land and water are looked after by everyone in today’s society, so that tomorrow’s generations inherit a cleaner, healthier world. Our work includes tackling flooding and pollution incidents, reducing industry’s impacts on the environment, cleaning up rivers, coastal waters and contaminated land, and improving wildlife habitats. This report is the result of research commissioned and funded by the Environment Agency (Habitats Directive Programme), English Nature and the Countryside Council for Wales. Published by: Author: Environment Agency, Rio House, Waterside Drive, Aztec West, N. Giles Almondsbury, Bristol, BS32 4UD Tel: 01454 624400 Fax: 01454 624409 Dissemination Status: www.environment-agency.gov.uk Publicly available ISBN: 978-1-84432-796-6 Keywords: Trout, stocking, cSAC rivers, salmon, bullhead, crayfish © Environment Agency July 2007 Research Contractor: All rights reserved. This document may be reproduced with prior Dr Nick Giles & Associates, permission of the Environment Agency. 50 Lake Road, Verwood, Dorset, BH31 6BX. The views expressed in this document are not necessarily Tel: 01202 824245 those of the Environment Agency. Email: [email protected] This report is printed on Cyclus Print, a 100% recycled stock, Environment Agency’s Project Manager: which is 100% post consumer waste and is totally chlorine free. Miran Aprahamian, Richard Fairclough House, Warrington Water used is treated and in most cases returned to source in better condition than removed. -

The Neurotoxin Β-N-Methylamino-L-Alanine (BMAA)
The neurotoxin β-N-methylamino-L-alanine (BMAA) Sources, bioaccumulation and extraction procedures Sandra Ferreira Lage ©Sandra Ferreira Lage, Stockholm University 2016 Cover image: Cyanobacteria, diatoms and dinoflagellates microscopic pictures taken by Sandra Ferreira Lage ISBN 978-91-7649-455-4 Printed in Sweden by Holmbergs, Malmö 2016 Distributor: Department of Ecology, Environment and Plant Sciences, Stockholm University “Sinto mais longe o passado, sinto a saudade mais perto.” Fernando Pessoa, 1914. Abstract β-methylamino-L-alanine (BMAA) is a neurotoxin linked to neurodegeneration, which is manifested in the devastating human diseases amyotrophic lateral sclerosis, Alzheimer’s and Parkinson’s disease. This neurotoxin is known to be produced by almost all tested species within the cyanobacterial phylum including free living as well as the symbiotic strains. The global distribution of the BMAA producers ranges from a terrestrial ecosystem on the Island of Guam in the Pacific Ocean to an aquatic ecosystem in Northern Europe, the Baltic Sea, where annually massive surface blooms occur. BMAA had been shown to accumulate in the Baltic Sea food web, with highest levels in the bottom dwelling fish-species as well as in mollusks. One of the aims of this thesis was to test the bottom-dwelling bioaccumulation hy- pothesis by using a larger number of samples allowing a statistical evaluation. Hence, a large set of fish individuals from the lake Finjasjön, were caught and the BMAA concentrations in different tissues were related to the season of catching, fish gender, total weight and species. The results reveal that fish total weight and fish species were positively correlated with BMAA concentration in the fish brain. -

Beyond Fish Edna Metabarcoding: Field Replicates Disproportionately Improve the Detection of Stream Associated Vertebrate Specie
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.26.437227; this version posted March 26, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 2 3 Beyond fish eDNA metabarcoding: Field replicates 4 disproportionately improve the detection of stream 5 associated vertebrate species 6 7 8 9 Till-Hendrik Macher1, Robin Schütz1, Jens Arle2, Arne J. Beermann1,3, Jan 10 Koschorreck2, Florian Leese1,3 11 12 13 1 University of Duisburg-Essen, Aquatic Ecosystem Research, Universitätsstr. 5, 45141 Essen, 14 Germany 15 2German Environmental Agency, Wörlitzer Platz 1, 06844 Dessau-Roßlau, Germany 16 3University of Duisburg-Essen, Centre for Water and Environmental Research (ZWU), Universitätsstr. 17 3, 45141 Essen, Germany 18 19 20 21 22 Keywords: birds, biomonitoring, bycatch, conservation, environmental DNA, mammals 23 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.03.26.437227; this version posted March 26, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 24 Abstract 25 Fast, reliable, and comprehensive biodiversity monitoring data are needed for 26 environmental decision making and management. Recent work on fish environmental 27 DNA (eDNA) metabarcoding shows that aquatic diversity can be captured fast, reliably, 28 and non-invasively at moderate costs. -

Paleolithic Fish from Southern Poland: a Paleozoogeographical Approach
10. ARCH. VOL. 22 (2ª)_ARCHAEOFAUNA 04/09/13 18:05 Página 123 Archaeofauna 22 (2013): 123-131 Paleolithic Fish from Southern Poland: A Paleozoogeographical Approach LEMBI LÕUGAS1, PIOTR WOJTAL2, JAROSŁAW WILCZYń SKI2 & KRZYSZTOF STEFANIAK3 1Department of Archaeobiology and Ancient Technology, Institute of History, University of Tallinn, Rüütli 6, EE10130 Tallinn, Estonia [email protected] 2Institute of Systematics and Evolution of Animals, Polish Academy of Sciences, Slawkowska 17, 31-016 Cracow, Poland [email protected], [email protected] 3Institute of Zoology, University of Wrocław, Sienkiewicza 21, 50-335 Wrocław, Poland [email protected] (Received 5 August 2012; Revised 31 October 2012; Accepted 17 July 2013) ABSTRACT: The area covered by glaciers during the Last Glacial Maximum (LGM) includes a large territory in northern Europe. In this region, Paleolithic finds are rare and fish bones fair- ly unique. Analysis of Paleolithic fish bones outside of the LGM range was carried out with the intention of reconstructing the paleozoogeographical distribution of this animal group before the retreat of the ice cap from the Baltic Basin. This research focuses on an archaeological fish bone assemblage from Obłazowa Cave, southern Poland. Other samples examined are from Krucza Skała Rock Shelter (Kroczyckie Rocks), Biśnik Cave (Wodąca Valley), Borsuka Cave (Szklarka Valley), and Nad Tunelem Cave (Prądnik Valley). The latter sites are considered natu- rally accumulated deposits, but, at Obłazowa and Krucza Skała, anthropogenic factors also played an important role. The fish bones from the Paleolithic cave deposits of Obłazowa inclu- ded at least six fish genera: Thymallus, Coregonus, Salmo, Salvelinus, Esox, and Cottus. -
Labidesthes Sicculus
Version 2, 2015 United States Fish and Wildlife Service Lower Great Lakes Fish and Wildlife Conservation Office 1 Atherinidae Atherinidae Sand Smelt Distinguishing Features: — (Atherina boyeri) — Sand Smelt (Non-native) Old World Silversides Old World Silversides Old World (Atherina boyeri) Two widely separated dorsal fins Eye wider than Silver color snout length 39-49 lateral line scales 2 anal spines, 13-15.5 rays Rainbow Smelt (Non -Native) (Osmerus mordax) No dorsal spines Pale green dorsally Single dorsal with adipose fin Coloring: Silver Elongated, pointed snout No anal spines Size: Length: up to 145mm SL Pink/purple/blue iridescence on sides Distinguishing Features: Dorsal spines (total): 7-10 Brook Silverside (Native) 1 spine, 10-11 rays Dorsal soft rays (total): 8-16 (Labidesthes sicculus) 4 spines Anal spines: 2 Anal soft rays: 13-15.5 Eye diameter wider than snout length Habitat: Pelagic in lakes, slow or still waters Similar Species: Rainbow Smelt (Osmerus mordax), 75-80 lateral line scales Brook Silverside (Labidesthes sicculus) Elongated anal fin Images are not to scale 2 3 Centrarchidae Centrarchidae Redear Sunfish Distinguishing Features: (Lepomis microlophus) Redear Sunfish (Non-native) — — Sunfishes (Lepomis microlophus) Sunfishes Red on opercular flap No iridescent lines on cheek Long, pointed pectoral fins Bluegill (Native) Dark blotch at base (Lepomis macrochirus) of dorsal fin No red on opercular flap Coloring: Brownish-green to gray Blue-purple iridescence on cheek Bright red outer margin on opercular flap -

Commercial Inland Fishing in Member Countries of the European Inland Fisheries Advisory Commission (EIFAC)
Commercial inland fishing in member countries of the European Inland Fisheries Advisory Commission (EIFAC): Operational environments, property rights regimes and socio-economic indicators Country Profiles May 2010 Mitchell, M., Vanberg, J. & Sipponen, M. EIFAC Ad Hoc Working Party on Socio-Economic Aspects of Inland Fisheries The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views of FAO. All rights reserved. FAO encourages the reproduction and dissemination of material in this information product. Non-commercial uses will be authorized free of charge, upon request. Reproduction for resale or other commercial purposes, including educational purposes, may incur fees. Applications for permission to reproduce or disseminate FAO copyright materials, and all queries concerning rights and licences, should be addressed by e-mail to [email protected] or to the Chief, Publishing Policy and Support Branch, Office of Knowledge Exchange, Research and Extension, FAO, Viale delle Terme di Caracalla, 00153 Rome, Italy. © FAO 2012 All papers have been reproduced as submitted. -

Beyond Fish Edna Metabarcoding: Field Replicates Disproportionately Improve the Detection of Stream Associated Vertebrate Species
Metabarcoding and Metagenomics 5: 59–71 DOI 10.3897/mbmg.5.66557 Research Article Beyond fish eDNA metabarcoding: Field replicates disproportionately improve the detection of stream associated vertebrate species Till-Hendrik Macher1, Robin Schütz1, Jens Arle2, Arne J. Beermann1,3, Jan Koschorreck2, Florian Leese1,3 1 University of Duisburg-Essen, Aquatic Ecosystem Research, Universitätsstr. 5, 45141 Essen, Germany 2 German Environment Agency, Wörlitzer Platz 1, 06844 Dessau-Roßlau, Germany 3 University of Duisburg-Essen, Centre for Water and Environmental Research (ZWU), Universitätsstr. 3, 45141 Essen, Germany Corresponding author: Till-Hendrik Macher ([email protected]) Academic editor: Pieter Boets | Received 26 March 2021 | Accepted 10 June 2021 | Published 13 July 2021 Abstract Fast, reliable, and comprehensive biodiversity monitoring data are needed for environmental decision making and management. Recent work on fish environmental DNA (eDNA) metabarcoding shows that aquatic diversity can be captured fast, reliably, and non-invasively at moderate costs. Because water in a catchment flows to the lowest point in the landscape, often a stream, it can col- lect traces of terrestrial species via surface or subsurface runoff along its way or when specimens come into direct contact with water (e.g., when drinking). Thus, fish eDNA metabarcoding data can provide information on fish but also on other vertebrate species that live in riparian habitats. This additional data may offer a much more comprehensive approach for assessing vertebrate diversity at no additional costs. Studies on how the sampling strategy affects species detection especially of stream-associated communities, however, are scarce. We therefore performed an analysis on the effects of biological replication on both fish as well as (semi-)terrestrial species detection. -

Identification of Priority Areas for the Conservation of Stream Fish Assemblages: Implications for River Management in France A
Identification of Priority Areas for the Conservation of Stream Fish Assemblages: Implications for River Management in France A. Maire, P. Laffaille, J.F. Maire, L. Buisson To cite this version: A. Maire, P. Laffaille, J.F. Maire, L. Buisson. Identification of Priority Areas for the Conservation of Stream Fish Assemblages: Implications for River Management in France. River Research and Applications, Wiley, 2016, 33 (4), pp.524-537. 10.1002/rra.3107. hal-01426354 HAL Id: hal-01426354 https://hal.archives-ouvertes.fr/hal-01426354 Submitted on 2 Jul 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License IDENTIFICATION OF PRIORITY AREAS FOR THE CONSERVATION OF STREAM FISH ASSEMBLAGES: IMPLICATIONS FOR RIVER MANAGEMENT IN FRANCE A. MAIREa*,†, P. LAFFAILLEb,c, J.-F. MAIREd AND L. BUISSONb,e a Irstea; UR HYAX, Pôle Onema-Irstea Hydroécologie des plans d’eau; Centre d’Aix-en-Provence, Aix-en-Provence, France b CNRS; UMR 5245 EcoLab, (Laboratoire Ecologie Fonctionnelle et Environnement), Toulouse, France c Université de Toulouse, INP, UPS; EcoLab; ENSAT, Castanet Tolosan, France d ONERA, The French Aerospace Lab Composites Department, Châtillon, France e Université de Toulouse, INP, UPS; EcoLab, Toulouse, France ABSTRACT Financial and human resources allocated to biodiversity conservation are often limited, making it impossible to protect all natural places, and priority areas for protection must be identified. -
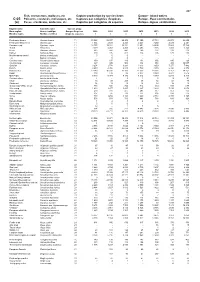
Fish, Crustaceans, Molluscs, Etc Capture Production by Species Items Europe
467 Fish, crustaceans, molluscs, etc Capture production by species items Europe - Inland waters C-05 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Europe - Eaux continentales (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Europa - Aguas continentales English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2009 2010 2011 2012 2013 2014 2015 Nombre inglés Nombre científico Grupo de especies t t t t t t t Freshwater bream Abramis brama 11 33 823 35 937 29 408 31 390 27 734 28 978 28 655 Freshwater breams nei Abramis spp 11 1 934 2 029 2 223 2 243 3 250 2 439 2 241 Common carp Cyprinus carpio 11 12 755 13 381 13 351 13 951 14 695 17 883 17 729 Tench Tinca tinca 11 3 019 4 053 2 455 2 253 1 686 1 491 1 328 Bleak Alburnus alburnus 11 682 547 441 269 231 213 219 Barbel Barbus barbus 11 300 195 412 224 205 170 188 Mediterranean barbel Barbus meridionalis 11 0 0 0 - - - - ...A Barbus cyclolepis 11 ... 0 0 - - - - Common nase Chondrostoma nasus 11 159 137 189 134 156 195 123 Crucian carp Carassius carassius 11 367 345 506 332 351 305 19 057 Goldfish Carassius auratus 11 3 254 2 778 3 293 3 613 3 653 7 277 7 482 Roach Rutilus rutilus 11 4 259 4 956 4 915 3 789 3 670 6 328 6 378 Roaches nei Rutilus spp 11 13 243 16 331 17 160 17 553 18 361 17 757 14 151 Rudd Scardinius erythrophthalmus 11 108 139 96 8 077 9 380 8 977 7 516 Orfe(=Ide) Leuciscus idus 11 5 040 5 374 5 330 4 812 5 979 6 240 5 316 Common dace Leuciscus leuciscus 11 4 .. -

(KHV) from Potential Vector Species to Common Carp
212, Bull. Eur. Ass. Fish Pathol., 32(6) 2012 Horizontal transmission of koi herpes virus (KHV) from potential vector species to common carp J. Kempter1ǰȱǯȱ ÙÚ1, R. Panicz1*, J. Sadowski1, ǯȱ¢Ù 1 and S. M. Bergmann2 ŗȱȱȱǰȱ¢ȱȱȱȱȱǰȱȱȱ ¢ȱȱ¢ȱȱ££ǰȱ ££ȱ à £ȱŚǰȱŝŗȬśśŖȱ££DzȱŘȱȬ ĝȬ ȱǻ Ǽǰȱȱȱ ȱȱȱ ǰȱ ȱȱ ¢ȱȱ ǰȱûŗŖǰȱŗŝŚşřȱ Ȭȱ ȱǰȱ ¢ Abstract ¡ȱęȱǰȱęȱȱȱȱȱȱȱȱǻ Ǽȱǰȱ¢DZȱ ȱǰȱȱǰȱǰȱȱěǰȱȱȱȱȱȱ ȱȱȱ ȱ¢ǯȱȱęȱȱȱȱȱȱȱȱęȱȱ¢ȱ ȱ ȱȱ been diagnosed and prior to the beginning of the research study the presence of the virus genome ȱęȱȱȱȱęȱȱȱǯȱęȱȱȱǻǼȱȱ utilized in the experiment originated from the University of Wageningen. During a four-week period the SPF carp were exposed to infection through cohabitation with vector species previously ęȱȱ ȱǯȱȱȱȱȱȱ¢ȱȱȱ£ȱ- mission of KHV between selected species, even in the case of species showing no clinical signs of KHV disease (KHVD), while an average water temperature in the tanks ranged from 12°C to 16°C. Introduction Koi herpes virus (KHV) disease (KHVD) is a free) SPF common carp (Bergmann et al., 2010). serious viral disease causing mass mortality in The possibilities of the virus being transferred the species ¢ȱ. According to pre- ¢ȱęȱȱȱȱȱ ȱ¢ȱ vious research, clinical signs can be observed as part of an experimental study performed by not only in common carp (ǯȱ), but also Kempter et al. (2008). According to the results, in hybrids: ǯȱ x ȱ and ǯȱ during experimental infection by immersion, x ȱ. Immersion aquaria species such as tench (ȱ), vimba (ȱ challenge experiments, showed severe losses ), common bream (ȱ) and Ğȱȱ ȱ ȱȱȱ ȱ grass carp (¢ȱ) can transmit řśƖȱȱŗŖŖƖȱȱȱ¡ȱęȱȱȱ¡ȱȱ KHV to SPF carp when water temperatures ȱ¢ȱȱ ȱȱȱǻęȬȬ range between 18 and 27°C.