A New Database Dedicated to the Reproduction of Temperate Freshwater Teleost Fishes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Beyond Fish Edna Metabarcoding: Field Replicates Disproportionately Improve the Detection of Stream Associated Vertebrate Specie
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.26.437227; this version posted March 26, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 2 3 Beyond fish eDNA metabarcoding: Field replicates 4 disproportionately improve the detection of stream 5 associated vertebrate species 6 7 8 9 Till-Hendrik Macher1, Robin Schütz1, Jens Arle2, Arne J. Beermann1,3, Jan 10 Koschorreck2, Florian Leese1,3 11 12 13 1 University of Duisburg-Essen, Aquatic Ecosystem Research, Universitätsstr. 5, 45141 Essen, 14 Germany 15 2German Environmental Agency, Wörlitzer Platz 1, 06844 Dessau-Roßlau, Germany 16 3University of Duisburg-Essen, Centre for Water and Environmental Research (ZWU), Universitätsstr. 17 3, 45141 Essen, Germany 18 19 20 21 22 Keywords: birds, biomonitoring, bycatch, conservation, environmental DNA, mammals 23 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.03.26.437227; this version posted March 26, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 24 Abstract 25 Fast, reliable, and comprehensive biodiversity monitoring data are needed for 26 environmental decision making and management. Recent work on fish environmental 27 DNA (eDNA) metabarcoding shows that aquatic diversity can be captured fast, reliably, 28 and non-invasively at moderate costs. -

Labidesthes Sicculus
Version 2, 2015 United States Fish and Wildlife Service Lower Great Lakes Fish and Wildlife Conservation Office 1 Atherinidae Atherinidae Sand Smelt Distinguishing Features: — (Atherina boyeri) — Sand Smelt (Non-native) Old World Silversides Old World Silversides Old World (Atherina boyeri) Two widely separated dorsal fins Eye wider than Silver color snout length 39-49 lateral line scales 2 anal spines, 13-15.5 rays Rainbow Smelt (Non -Native) (Osmerus mordax) No dorsal spines Pale green dorsally Single dorsal with adipose fin Coloring: Silver Elongated, pointed snout No anal spines Size: Length: up to 145mm SL Pink/purple/blue iridescence on sides Distinguishing Features: Dorsal spines (total): 7-10 Brook Silverside (Native) 1 spine, 10-11 rays Dorsal soft rays (total): 8-16 (Labidesthes sicculus) 4 spines Anal spines: 2 Anal soft rays: 13-15.5 Eye diameter wider than snout length Habitat: Pelagic in lakes, slow or still waters Similar Species: Rainbow Smelt (Osmerus mordax), 75-80 lateral line scales Brook Silverside (Labidesthes sicculus) Elongated anal fin Images are not to scale 2 3 Centrarchidae Centrarchidae Redear Sunfish Distinguishing Features: (Lepomis microlophus) Redear Sunfish (Non-native) — — Sunfishes (Lepomis microlophus) Sunfishes Red on opercular flap No iridescent lines on cheek Long, pointed pectoral fins Bluegill (Native) Dark blotch at base (Lepomis macrochirus) of dorsal fin No red on opercular flap Coloring: Brownish-green to gray Blue-purple iridescence on cheek Bright red outer margin on opercular flap -

Beyond Fish Edna Metabarcoding: Field Replicates Disproportionately Improve the Detection of Stream Associated Vertebrate Species
Metabarcoding and Metagenomics 5: 59–71 DOI 10.3897/mbmg.5.66557 Research Article Beyond fish eDNA metabarcoding: Field replicates disproportionately improve the detection of stream associated vertebrate species Till-Hendrik Macher1, Robin Schütz1, Jens Arle2, Arne J. Beermann1,3, Jan Koschorreck2, Florian Leese1,3 1 University of Duisburg-Essen, Aquatic Ecosystem Research, Universitätsstr. 5, 45141 Essen, Germany 2 German Environment Agency, Wörlitzer Platz 1, 06844 Dessau-Roßlau, Germany 3 University of Duisburg-Essen, Centre for Water and Environmental Research (ZWU), Universitätsstr. 3, 45141 Essen, Germany Corresponding author: Till-Hendrik Macher ([email protected]) Academic editor: Pieter Boets | Received 26 March 2021 | Accepted 10 June 2021 | Published 13 July 2021 Abstract Fast, reliable, and comprehensive biodiversity monitoring data are needed for environmental decision making and management. Recent work on fish environmental DNA (eDNA) metabarcoding shows that aquatic diversity can be captured fast, reliably, and non-invasively at moderate costs. Because water in a catchment flows to the lowest point in the landscape, often a stream, it can col- lect traces of terrestrial species via surface or subsurface runoff along its way or when specimens come into direct contact with water (e.g., when drinking). Thus, fish eDNA metabarcoding data can provide information on fish but also on other vertebrate species that live in riparian habitats. This additional data may offer a much more comprehensive approach for assessing vertebrate diversity at no additional costs. Studies on how the sampling strategy affects species detection especially of stream-associated communities, however, are scarce. We therefore performed an analysis on the effects of biological replication on both fish as well as (semi-)terrestrial species detection. -

Identification of Priority Areas for the Conservation of Stream Fish Assemblages: Implications for River Management in France A
Identification of Priority Areas for the Conservation of Stream Fish Assemblages: Implications for River Management in France A. Maire, P. Laffaille, J.F. Maire, L. Buisson To cite this version: A. Maire, P. Laffaille, J.F. Maire, L. Buisson. Identification of Priority Areas for the Conservation of Stream Fish Assemblages: Implications for River Management in France. River Research and Applications, Wiley, 2016, 33 (4), pp.524-537. 10.1002/rra.3107. hal-01426354 HAL Id: hal-01426354 https://hal.archives-ouvertes.fr/hal-01426354 Submitted on 2 Jul 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License IDENTIFICATION OF PRIORITY AREAS FOR THE CONSERVATION OF STREAM FISH ASSEMBLAGES: IMPLICATIONS FOR RIVER MANAGEMENT IN FRANCE A. MAIREa*,†, P. LAFFAILLEb,c, J.-F. MAIREd AND L. BUISSONb,e a Irstea; UR HYAX, Pôle Onema-Irstea Hydroécologie des plans d’eau; Centre d’Aix-en-Provence, Aix-en-Provence, France b CNRS; UMR 5245 EcoLab, (Laboratoire Ecologie Fonctionnelle et Environnement), Toulouse, France c Université de Toulouse, INP, UPS; EcoLab; ENSAT, Castanet Tolosan, France d ONERA, The French Aerospace Lab Composites Department, Châtillon, France e Université de Toulouse, INP, UPS; EcoLab, Toulouse, France ABSTRACT Financial and human resources allocated to biodiversity conservation are often limited, making it impossible to protect all natural places, and priority areas for protection must be identified. -
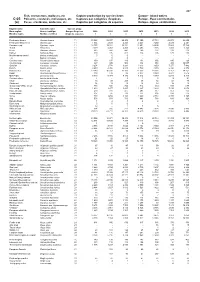
Fish, Crustaceans, Molluscs, Etc Capture Production by Species Items Europe
467 Fish, crustaceans, molluscs, etc Capture production by species items Europe - Inland waters C-05 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Europe - Eaux continentales (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Europa - Aguas continentales English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2009 2010 2011 2012 2013 2014 2015 Nombre inglés Nombre científico Grupo de especies t t t t t t t Freshwater bream Abramis brama 11 33 823 35 937 29 408 31 390 27 734 28 978 28 655 Freshwater breams nei Abramis spp 11 1 934 2 029 2 223 2 243 3 250 2 439 2 241 Common carp Cyprinus carpio 11 12 755 13 381 13 351 13 951 14 695 17 883 17 729 Tench Tinca tinca 11 3 019 4 053 2 455 2 253 1 686 1 491 1 328 Bleak Alburnus alburnus 11 682 547 441 269 231 213 219 Barbel Barbus barbus 11 300 195 412 224 205 170 188 Mediterranean barbel Barbus meridionalis 11 0 0 0 - - - - ...A Barbus cyclolepis 11 ... 0 0 - - - - Common nase Chondrostoma nasus 11 159 137 189 134 156 195 123 Crucian carp Carassius carassius 11 367 345 506 332 351 305 19 057 Goldfish Carassius auratus 11 3 254 2 778 3 293 3 613 3 653 7 277 7 482 Roach Rutilus rutilus 11 4 259 4 956 4 915 3 789 3 670 6 328 6 378 Roaches nei Rutilus spp 11 13 243 16 331 17 160 17 553 18 361 17 757 14 151 Rudd Scardinius erythrophthalmus 11 108 139 96 8 077 9 380 8 977 7 516 Orfe(=Ide) Leuciscus idus 11 5 040 5 374 5 330 4 812 5 979 6 240 5 316 Common dace Leuciscus leuciscus 11 4 .. -

And Perca Fluviatilis
International Journal of Environmental Research and Public Health Article Using Rutilus rutilus (L.) and Perca fluviatilis (L.) as Bioindicators of the Environmental Condition and Human Health: Lake Ła ´nskie,Poland Joanna Łuczy ´nska 1,* , Beata Paszczyk 1, Marek Jan Łuczy ´nski 2, Monika Kowalska-Góralska 3 , Joanna Nowosad 4 and Dariusz Kucharczyk 4 1 Chair of Commodity and Food Analysis, University of Warmia and Mazury in Olsztyn, ul. Plac Cieszy´nski1, 10-726 Olsztyn, Poland; [email protected] 2 The Stanisław Sakowicz Inland Fisheries Institute in Olsztyn, ul. Oczapowskiego 10, 10-719 Olsztyn, Poland; mj.luczynski@infish.com.pl 3 Department of Limnology and Fishery, Institute of Animal Breeding, Faculty of Biology and Animal Science, Wrocław University of Environmental and Life Sciences, ul. J. Chełmo´nskiego38 c, 51-630 Wrocław, Poland; [email protected] 4 Department of Ichthyology and Aquaculture, Warmia and Mazury University, Al. Warszawska 117A, 10-701 Olsztyn, Poland; [email protected] (J.N.); [email protected] (D.K.) * Correspondence: [email protected]; Tel.: +48-89523-4165 Received: 15 September 2020; Accepted: 16 October 2020; Published: 19 October 2020 Abstract: The aim of this study was to determine the mercury content and fatty acids profile in roach (Rutilus rutilus L.) and European perch (Perca fluviatilis L.) from Lake Ła´nskie(Poland). Mercury content was higher in the muscles than other organs in both species (p < 0.05). Mercury accumulates along the food chain of the lake’s ecosystem. The value of the bioconcentration factor (BCF) indicated that Hg had accumulated in the highest amounts in muscles and in the other organs as follows: muscles > liver > gills > gonads. -

Applied Freshwater Fish Biology an Introduction to Methods of Research and Management
How to preserve and how to exploit natural populations to be sustained for the future. Plain questions without equally plain answers Applied freshwater fish biology An introduction to methods of research and management Arne N. Linløkken, ass. professor Inland Norway University of Applied Sciences Arne N. 1 CONTENT INTRODUCTION .................................................................................................................................................. 3 Prehistory and evolution ......................................................................................................................................... 3 Short on construction and function ......................................................................................................................... 4 Morphology ........................................................................................................................................................ 4 Anatomy and physiology .................................................................................................................................... 5 European freshwater fish species ............................................................................................................................ 6 Immigration and distribution of freshwater fish in western Scandinavia ........................................................... 7 Western immigrants ........................................................................................................................ 8 -

Assessing the Species in the CARES Preservation Program and the Role of Aquarium Hobbyists in Freshwater Fish Conservation
fishes Article Assessing the Species in the CARES Preservation Program and the Role of Aquarium Hobbyists in Freshwater Fish Conservation Jose W. Valdez 1 and Kapil Mandrekar 2,* 1 Department of Bioscience—Biodiversity and Conservation, Aarhus University, Grenåvej 14, 8410 Rønde, Denmark; [email protected] 2 Department of Environmental and Forest Biology, SUNY College of Environmental Science and Forestry, 1 Forestry Drive, Syracuse, NY 13210, USA * Correspondence: [email protected] Received: 30 June 2019; Accepted: 17 September 2019; Published: 29 September 2019 Abstract: Freshwater fish represent half of all fish species and are the most threatened vertebrate group. Given their considerable passion and knowledge, aquarium hobbyists can play a vital role in their conservation. CARES is made up of many organizations, whose purpose is to encourage aquarium hobbyists to devote tank space to the most endangered and overlooked freshwater fish to ensure their survival. We found the CARES priority list contains nearly six hundred species from twenty families and two dozen extinct-in-the-wild species. The major families were typically those with the largest hobbyist affiliations such as killifish, livebearers, and cichlids, the latter containing half of CARES species. CARES included every IUCN threatened species of Pseudomugilidae and Valenciidae, but only one percent of threatened Characidae, Cobitidae, and Gobiidae species. No Loricariidae in CARES were in the IUCN red list as they have not been scientifically described. Tanzania and Mexico contained the largest amount of species, with the latter containing the most endemics. Many species were classified differently than the IUCN, including a third of extinct-in-the-wild species classified as least concern by the IUCN. -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste -

The Living Planet Index (Lpi) for Migratory Freshwater Fish Technical Report
THE LIVING PLANET INDEX (LPI) FOR MIGRATORY FRESHWATER FISH LIVING PLANET INDEX TECHNICAL1 REPORT LIVING PLANET INDEXTECHNICAL REPORT ACKNOWLEDGEMENTS We are very grateful to a number of individuals and organisations who have worked with the LPD and/or shared their data. A full list of all partners and collaborators can be found on the LPI website. 2 INDEX TABLE OF CONTENTS Stefanie Deinet1, Kate Scott-Gatty1, Hannah Rotton1, PREFERRED CITATION 2 1 1 Deinet, S., Scott-Gatty, K., Rotton, H., Twardek, W. M., William M. Twardek , Valentina Marconi , Louise McRae , 5 GLOSSARY Lee J. Baumgartner3, Kerry Brink4, Julie E. Claussen5, Marconi, V., McRae, L., Baumgartner, L. J., Brink, K., Steven J. Cooke2, William Darwall6, Britas Klemens Claussen, J. E., Cooke, S. J., Darwall, W., Eriksson, B. K., Garcia Eriksson7, Carlos Garcia de Leaniz8, Zeb Hogan9, Joshua de Leaniz, C., Hogan, Z., Royte, J., Silva, L. G. M., Thieme, 6 SUMMARY 10 11, 12 13 M. L., Tickner, D., Waldman, J., Wanningen, H., Weyl, O. L. Royte , Luiz G. M. Silva , Michele L. Thieme , David Tickner14, John Waldman15, 16, Herman Wanningen4, Olaf F., Berkhuysen, A. (2020) The Living Planet Index (LPI) for 8 INTRODUCTION L. F. Weyl17, 18 , and Arjan Berkhuysen4 migratory freshwater fish - Technical Report. World Fish Migration Foundation, The Netherlands. 1 Indicators & Assessments Unit, Institute of Zoology, Zoological Society 11 RESULTS AND DISCUSSION of London, United Kingdom Edited by Mark van Heukelum 11 Data set 2 Fish Ecology and Conservation Physiology Laboratory, Department of Design Shapeshifter.nl Biology and Institute of Environmental Science, Carleton University, Drawings Jeroen Helmer 12 Global trend Ottawa, ON, Canada 15 Tropical and temperate zones 3 Institute for Land, Water and Society, Charles Sturt University, Albury, Photography We gratefully acknowledge all of the 17 Regions New South Wales, Australia photographers who gave us permission 20 Migration categories 4 World Fish Migration Foundation, The Netherlands to use their photographic material. -

Lagoon Resident Fish Species of Conservation Interest According to the Habitat Directive (92/43/CEE): a Review on Their Potential Use As Ecological Indicator Species
water Review Lagoon Resident Fish Species of Conservation Interest According to the Habitat Directive (92/43/CEE): A Review on Their Potential Use as Ecological Indicator Species Chiara Facca * , Francesco Cavraro, Piero Franzoi and Stefano Malavasi Department of Environmental Sciences, Informatics and Statistics, Ca’ Foscari University of Venice, via Torino 155, 30175 Venice, Italy; [email protected] (F.C.); [email protected] (P.F.); [email protected] (S.M.) * Correspondence: [email protected]; Tel.: +39-(0)-41-234-7733 Received: 22 May 2020; Accepted: 17 July 2020; Published: 20 July 2020 Abstract: Transitional waters are fragile ecosystems with high ecological, social and economic values, that undergo numerous threats. According to the information provided by European Member States in the framework of the European Directive 92/43/EEC (Habitat Directive), the main threat to these ecosystems is represented by morphological and hydrological changes. The present work focuses on six lagoon fish species included in the Habitat Directive annex II (species requiring conservation measures: Aphanius fasciatus, A. iberus, Knipowitschia panizzae, Ninnigobius canestrinii, Valencia hispanica and V. letourneuxi) that spend their entire life cycle in the Mediterranean priority habitat 1150* “Coastal lagoons”. The overview of the current scientific literature allowed us to highlight how the presence and abundance of these species may provide important indications on the conservation status of coastal lagoon habitats. In fact, their occurrence, distribution and biology depend on the presence of peculiar structures, such as salt marshes, small channels, isolated pools and oligohaline areas. Coastal lagoon fragmentation and habitat loss have led to a significant reduction in genetic diversity or local population extinction. -

Effects of Enhanced Hydrological Connectivity on Mediterranean Salt Marsh fish Assemblages with Emphasis on the Endangered Spanish Toothcarp (Aphanius Iberus)
Effects of enhanced hydrological connectivity on Mediterranean salt marsh fish assemblages with emphasis on the endangered Spanish toothcarp (Aphanius iberus) Patricia Prado, Carles Alcaraz, Lluis Jornet, Nuno Caiola and Carles Iba´n˜ez Aquatic Ecosystems, Institut de Recerca i Tecnologia Agroalimenta`ries, Sant Carles de la Ra`pita, Tarragona, Spain ABSTRACT The hydrological connectivity between the salt marsh and the sea was partially restored in a Mediterranean wetland containing isolated ponds resulting from former salt extraction and aquaculture activities. A preliminary assessment provided evidence that ponds farther from the sea hosted very large numbers of the endangered Spanish toothcarp, Aphanius iberus, suggesting that individuals had been trapped and consequently reach unnaturally high densities. In order to achieve both habitat rehabilitation and toothcarp conservation, efforts were made to create a gradient of hydrologically connected areas, including isolated fish reservoirs, semi-isolated, and connected salt marsh-sea areas that could allow migratory movements of fish and provide some protection for A. iberus. The fish community was monitored prior to, and for three years after rehabilitation. Results showed an increase in the number of fish species within semi-isolated areas (Zone A), whereas areas adjacent to the sea (Zone B) increased the number of marine species and decreased that of estuarine species (ES). Yet overall differences in fish assemblages Submitted 9 October 2016 were much higher between zones than among study years. Generalized linear models Accepted 21 January 2017 (GLMs) evidenced that distance to the sea was the most important variable Published 28 February 2017 explaining the local diversity of the fish community after restoration, with Corresponding author occasional influence of other factors such as temperature, and depth.