Pollen Fidelity of Apis Mellifera L. During Pollination of Limnanthes Alba Hartw
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pollination and Evolution of Plant and Insect Interaction JPP 2017; 6(3): 304-311 Received: 03-03-2017 Accepted: 04-04-2017 Showket a Dar, Gh
Journal of Pharmacognosy and Phytochemistry 2017; 6(3): 304-311 E-ISSN: 2278-4136 P-ISSN: 2349-8234 Pollination and evolution of plant and insect interaction JPP 2017; 6(3): 304-311 Received: 03-03-2017 Accepted: 04-04-2017 Showket A Dar, Gh. I Hassan, Bilal A Padder, Ab R Wani and Sajad H Showket A Dar Parey Sher-e-Kashmir University of Agricultural Science and Technology, Shalimar, Jammu Abstract and Kashmir-India Flowers exploit insects to achieve pollination; at the same time insects exploit flowers for food. Insects and flowers are a partnership. Each insect group has evolved different sets of mouthparts to exploit the Gh. I Hassan food that flowers provide. From the insects' point of view collecting nectar or pollen is rather like fitting Sher-e-Kashmir University of a key into a lock; the mouthparts of each species can only exploit flowers of a certain size and shape. Agricultural Science and This is why, to support insect diversity in our gardens, we need to plant a diversity of suitable flowers. It Technology, Shalimar, Jammu is definitely not a case of 'one size fits all'. While some insects are generalists and can exploit a wide and Kashmir-India range of flowers, others are specialists and are quite particular in their needs. In flowering plants, pollen grains germinate to form pollen tubes that transport male gametes (sperm cells) to the egg cell in the Bilal A Padder embryo sac during sexual reproduction. Pollen tube biology is complex, presenting parallels with axon Sher-e-Kashmir University of guidance and moving cell systems in animals. -

Pollination of Cultivated Plants in the Tropics 111 Rrun.-Co Lcfcnow!Cdgmencle
ISSN 1010-1365 0 AGRICULTURAL Pollination of SERVICES cultivated plants BUL IN in the tropics 118 Food and Agriculture Organization of the United Nations FAO 6-lina AGRICULTUTZ4U. ionof SERNES cultivated plans in tetropics Edited by David W. Roubik Smithsonian Tropical Research Institute Balboa, Panama Food and Agriculture Organization of the United Nations F'Ø Rome, 1995 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. M-11 ISBN 92-5-103659-4 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the copyright owner. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Publications Division, Food and Agriculture Organization of the United Nations, Viale delle Terme di Caracalla, 00100 Rome, Italy. FAO 1995 PlELi. uion are ted PlauAr David W. Roubilli (edita Footli-anal ISgt-iieulture Organization of the Untled Nations Contributors Marco Accorti Makhdzir Mardan Istituto Sperimentale per la Zoologia Agraria Universiti Pertanian Malaysia Cascine del Ricci° Malaysian Bee Research Development Team 50125 Firenze, Italy 43400 Serdang, Selangor, Malaysia Stephen L. Buchmann John K. S. Mbaya United States Department of Agriculture National Beekeeping Station Carl Hayden Bee Research Center P. -
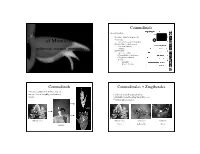
Diversity and Evolution of Monocots
Commelinids 4 main groups: Diversity and Evolution • Acorales - sister to all monocots • Alismatids of Monocots – inc. Aroids - jack in the pulpit • Lilioids (lilies, orchids, yams) – non-monophyletic . spiderworts, bananas, pineapples . – petaloid • Commelinids – Arecales – palms – Commelinales – spiderwort – Zingiberales –banana – Poales – pineapple – grasses & sedges Commelinids Commelinales + Zingiberales • theme: reduction of flower, loss of nectar, loss of zoophily, evolution of • 2 closely related tropical orders bracts • primarily nectar bearing but with losses • bracted inflorescences grass pickeral weed pickeral weed spiderwort heliconia nectar pollen only bracts rapatead bromeliad Commelinaceae - spiderwort Commelinaceae - spiderwort Family of small herbs with succulent stems, stems jointed; leaves sheathing. Family does not produce Inflorescence often bracted nectar, but showy flowers for insect pollen gathering. Rhoeo - Moses in a cradle Commelina erecta - Erect dayflower Tradescantia ohiensis - spiderwort Tradescantia ohiensis - spiderwort Commelinaceae - spiderwort Commelinaceae - spiderwort Flowers actinomorphic or • species rich in pantropics, CA 3 CO 3 A 6 G (3) zygomorphic especially Africa • floral diversity is enormous Commelina communis - day flower Tradescantia ohiensis - spiderwort Pontederiaceae - pickerel weed Pontederiaceae - pickerel weed Aquatic family of emergents or floaters. Pickerel weed has glossy heart-shaped leaves, Water hyacinth (Eichhornia) from superficially like Sagittaria but without net venation. -

Sexual Systems of Plants in a Brazilian Montane Forest
Floresta e Ambiente 2019; 26(Spec No 1): e20180394 https://doi.org/10.1590/2179-8087.039418 ISSN 2179-8087 (online) Original Article Conservation of Nature Sexual Systems of Plants in a Brazilian Montane Forest Monique Perini1 , Henrique Machado Dias2 , Sustanis Horn Kunz2 1Programa de Pós-graduação em Biologia Vegetal, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais – UFMG, Belo Horizonte/MG, Brasil 2Departamento de Ciências Florestais e da Madeira, Universidade Federal do Espírito Santo – UFES, Jerônimo Monteiro/ES, Brasil ABSTRACT In this study, vegetation reproduction has been investigated in order to understand aspects of speciation, structuring and composition of plant communities. Thus, we sought to characterize the frequency of sexual systems from species recorded in seed rain occurring in a tropical rainforest (Atlantic Forest) in Caparaó National Park, Espírito Santo State, Brazil. We collected the seed rain for twelve months, classified and recorded the species for: sexual system; pollination and dispersion syndrome; and fruit type. Then we measured the correlation between these attributes through correspondence analysis. Regarding sexual systems, 71% were hermaphrodites, 13% dioecious, and 11% monoecious. Hermaphrodites are best associated with pollination, dispersion and fruit types, represented by 65% of data variance. This study may contribute to elaborating management and conservation programs taking into account the interaction of plants with the local fauna. Keywords: seed rain, reproductive ecology, woody layer, Caparaó National Park, Atlantic Forest. Creative Commons License. All the contents of this journal, except where otherwise noted, is licensed under a Creative Commons Attribution License. 2/10 Perini M, Dias HM, Kunz SH Floresta e Ambiente 2019; 26(Spec No 1): e20180394 1. -

3. the Importance of Bees in Nature
3. THE IMPORTANCE OF BEES IN NATURE BEES AS PART OF ECOSYSTEMS Pollinators strongly influence ecological relationships, ecosystem conservation and stability, genetic variation in the plant community, floral diversity, specialization and evolution. Bees play an important, but little recognized role in most terrestrial ecosystems where there is green vegetation cover for at least 3 to 4 months each year. In tropical forests, savannah woodlands, mangrove, and in temperate deciduous forests, many species of plants and animals would not survive if bees were missing. This is because the production of seeds, nuts, berries and fruits are highly dependent on insect pollination, and among the pollinating insects, bees are the major pollinators. In rain forests, especially in high mountain forests where it is too cold for most bees, other pollinators like bats and birds play a greater role in plant pollination. In farmed areas, bees are needed for the pollination of many cultivated crops (see Chapter 7), and for maintaining biodiversity in ‘islands’ of non-cultivated areas. The main role of bees in the different ecosystems is their pollination work. Other animal species are connected with bees: either because they eat the brood or honey, pollen or wax, because they are parasitic to the bees, or simply because they live within the bees nest. WHAT IS POLLINATION? Pollination is transfer of pollen from the anther (the male part of the flower) to the stigma (the female part of the flower). Some plants can pollinate themselves: in this case, the pollen passes from the anther to the stigma inside the same flower, and this is called self-pollination. -

Reproductive Ecology and Pollen Representation Among Neotropical
GEB247.fm Page 359 Tuesday, June 5, 2001 3:53 PM Global Ecology & Biogeography (2001) 10, 359–367 RESEARCH LETTER ReproductiveBlackwell Science, Ltd ecology and pollen representation among neotropical trees MARK B. BUSH1 and ROBERT RIVERA21Department of Biological Sciences, Florida Institute of Technology, 150 W. University Blvd, Melbourne, Florida 32901, U.S.A., E-mail: mbush@fit.edu 2Department of Botany, Duke University, Durham, North Carolina 27708, U.S.A. ABSTRACT species or those zoophilous species exhibiting ‘messy’ pollination syndromes. Pollination mech- Three years of pollen trapping data from Barro anisms will predictably bias the fossil record Colorado Island, Panama, are compared with local against certain flower morphologies. These data vegetation inventories. Two hypotheses relating are of significance to those using the fossil pollen pollen representation to ‘messy’ pollination and record to reconstruct the timing and sequence of flower form are tested. Dioecious taxa were found angiosperm evolution. These data help prioritize to be over-represented in pollen spectra compared plants to be included in modern pollen reference with their occurrence in local forests. Similarly, collections and to focus the search for ‘unknown’ anemophilous and ‘messy’ pollination types were types on most-likely candidate families. found to be over-represented. While anemophilous taxa were the best dispersed pollen types, zoophilous Key words anemophily, Barro Colorado Island, taxa were also well-represented in dispersal classes dioecious, hermaphroditic, monoecious, Panama, of 20–40 m and > 40 m. Thus pollen arriving to pollen representation, pollination syndrome, trop- lake sediments is most likely to be from anemophilous ical rain forest. diversity of pollen in tropical pollen rain, it is INTRODUCTION probable that an unexpectedly large proportion The description of pollination syndromes (Faegri of pollen is coming from zoophilous taxa. -

Pollination Biology
Pollination Biology . real story of the birds & bees . and beetles, bugs, butterflies, bats Sexual Reproduction in Plants • Movement onto land is an issue for sexual reproduction in plants - unlike for animals • rely on movement of (1) pollen, (2) young embryo encased in a seed (or fruit), or (3) spores pollination biology seed dispersal Sexual Reproduction in Plants Pollination and seed/spore dispersal important aspects of biosystematics in plants: • Gene flow • Outcrossing vs. inbreeding • Reproductive isolation • Speciation spore dispersal • Co-speciation (coevolution) pollination biology seed dispersal Coevolution Coevolution – interactions between two different clades as selective forces on each other, resulting in adaptations that increase their interdependency Animal-flowering plant interaction is a classic example of coevolution: • Plants evolve elaborate methods to attract animal pollinators • Animals evolve specialized body parts and behaviors that aid plant pollination ! Coevolution109 • coevolution with pollinators often leads to convergence and divergence in flowers • best studied has been the phlox family: Polemoniaceae Fig. 1. Floral diversity in Polemoniaceae. (A) Leptosiphon aureus subsp. aureus; (B) Saltugilia splendens subsp. grantii; (C) Navarretia hamata subsp. hamata; (D) Leptosiphon montanus; (E) Phlox divaricata subsp. laphamii; (F) Cantua buxifolia; (G) Aliciellla latifolia subsp. latifolia; (H) Linanthus orcutii; (I) Saltugilia caruifolia; (J) Loeseliastrum schottii; (K) Cobaea scandens; (L) Eriastrum eremicum -

Pollination.Pdf
Pollination Pollinators. Source: USFS; Credit: Paul Mirocha. Carpenter bee with pollen collected fromNight-blooming cereus Pollination in angiosperms and gymnosperms is the process that transfers pollen grains, which contain the male gametes (sperm) to where the female gamete(s) are contained within the carpel;[1] in gymnosperms the pollen is directly applied to the ovule itself. The receptive part of the carpel is called a stigma in the flowers of angiosperms. The receptive part of the gymnosperm ovule is called the micropyle. The study of pollination brings together many disciplines, such as botany, horticulture, entomology, and ecology. The pollination process as an interaction between flower and vector was first addressed in the 18th century by Christian Konrad Sprengel. Pollination is a necessary step in the sexual reproduction of flowering plants, resulting in the production of offspring that are genetically diverse. It is important in horticulture and agriculture, because fruiting is dependent on fertilisation, which is the end result of pollination. Pollinators are responsible for assisting over 80% of the world's flowering plants. Without them, humans and wildlife would not have much to eat or look at. Animals that assist plants in their reproduction as pollinators include species of ants, bats, bees, beetles, birds, butterflies, flies, moths, wasps, as well as otherunusual animals. Wind and water also play a role in the pollination of many plants. Types Abiotic pollination refers to situations where pollination is mediated without the involvement of other organisms; these abiotic factors that achieve pollination include wind and gravity. Only 10% of flowering plants are pollinated without animal assistance.[2] The most common form, anemophily, is pollination bywind. -

Impact of Climate Change on Insect Pollination
13 Article Impact of Climate Change on Insect Journal of Development Economics and Pollination Management Research Studies (JDMS) 01(01), 1-12, July-September 2019 @Center for Development Economic Studies (CDES) Reprints and permissions http://www.cdes.org.in/ http://www.cdes.org.in/journal/ Dr.S.Swaminathan ABSTRACT Self- and Cross-pollinations (Auto- and Allogamy) are the two kinds of pollination in flowers. Cross-pollination is brought out by external agents like insects, rodents, bats, birds, wind and water. The Entomophily or insect pollination which was evident from the 100 million year old amber fossils is of wide occurrence in modern time. Around 80% of the modern flowering plant species are insect pollinated. Insects such as Bees, Butterflies, Beetles, Thrips and Flies are the well known pollinators of wild, agricultural and horticultural plant species. The Climate Change caused by accumulating atmospheric Green House Gases (GHG) such as Carbon-dioxide, rising Global Temperature, abnormal precipitation and drought, etc. seem to change the phenology of both flowering plants and insect pollinators thereby reducing the efficiency of the latter in pollination. Further, reductions in the insect population and flower production, the other impacts of climate change, will have a direct effect on pollination. The adverse consequence of the pollinatory role of the major group of pollinators, the insects, will certainly have a more negative impact on the agricultural, horticultural and other plant production and that will in turn affect the global economy in future. Key words: Cross-pollination, Entomophily, Green House Gases, Phenology. Introduction: Self- and Cross-pollination (Autogamy and Allogamy) are the two kinds of pollinations seen in flowering plants. -

Poster Session Tick-Borne Diseases
Poster session Tick-borne diseases Incidence and trend of disseminated stages of Lyme Borreliosis in the province of Brabant Wallon (Belgium): retrospective data of a reference center Y.E. Somassè1, V. Luyasu2, S.O. Vanwambeke3, A.R. Robert1 1Epidemiology and Biostatistics Unit, IREC & IRSS, Université catholique de Louvain, Brussels, Belgium 2 Clinique Saint-Pierre, Ottignies-Louvain-la-Neuve, Belgium 3 Department of Geography, Université catholique de Louvain, Louvain-la-Neuve, Belgium Background: Lyme Borreliosis (LB) is the most common vector-borne disease in Europe (Bacon 2008). Epidemiological serological data in Belgium show an increasing incidence since 1993 (Ducoffre 2008). However, this serological data does not allow measuring the rate of disseminated forms, which constitute the burden of the disease. Using the annual incidence of disseminated stages of LB from a reference hospital (Clinique Saint-Pierre, Ottignies-Louvain-la-Neuve), we aimed at describing the clinical and epidemiological features of cases over an 11-years period (1997-2007) Methods: Hospital cases records of all LB diagnosed from January 1st, 1997 to December 31th, 2007 were collected. To estimate the incidence of disseminated LB in Brabant Wallon, we reported annual cases to the number of people living in the catchment area of the hospital. Results: Ninety-eight (98) cases of LB were registered, representing a mean of 8.9 cases per year. All cases were diagnosed at second or later stages of the disease. No linear trend in incident cases was observed. Most of the cases (75%) were recorded between June and November. Neuroborreliosis was the dominant clinical form every year, with a mean of 76.5%, similarly to a previous report in 1989 (Bigaignon 1989). -

Functional Integration of Floral Plant Traits: Shape and Symmetry, Optical Signal, Reward and Reproduction in the Angiosperm Flower
Functional Integration of Floral Plant Traits: Shape and Symmetry, Optical Signal, Reward and Reproduction in the Angiosperm Flower Dissertation zur Erlangung des Doktorgrades (Dr. rer. nat.) der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn vorgelegt von Andreas Wilhelm Mues aus Kirchhellen Bonn, den 20. Januar 2020 1 2 Angefertigt mit Genehmigung der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn Erstgutachter: Prof. Dr. Maximilian Weigend, Universität Bonn Zweitgutachter: Prof. Dr. Eberhard Fischer, Universität Koblenz Tag der Promotion: 30. April 2020 Erscheinungsjahr: 2020 3 4 Acknowledgements I thank Prof. Dr. Maximilian Weigend, supervisor, for his guidance and support, and for giving me the opportunity to study the holistic subject of floral functional integration and plant-animal interaction. I am grateful for the experience and for the research agendas he entrusted to me: Working with the extensive Living Collections of Bonn Botanical Gardens was an honour, and I have learned a lot. I thank Prof. Dr. Eberhard Fisher, for agreeing to be my second supervisor, his advice and our shared passion for the plant world. I would like to thank many people of the Nees Institute and Bonn Botanical Gardens who contributed to this work and who gave me good memories of my years of study: I thank Lisabeth Hoff, Tianjun Liu, Luisa Sophie Nicolin and Simon Brauwers for their contribution in collecting shares of the raw data together with me, and for being eager students – especially counting pollen and ovule numbers and measuring nectar reward was a test of patience sometimes, and we have counted and measured a lot … Thank you! Special thanks go to Gardeners of the Bonn Botanical Gardens, for their constant support throughout the years, their love for the plant world in general and their commitment and care for the Living Collection: Klaus Mahlberg (Streptocarpus), Birgit Emde (carnivorous plants), Klaus Bahr (Geraniales), Bernd Reinken and Klaus Michael Neumann. -

Pollination Wheel
Why? e of pollinator? is typ ct th ttra ts a What? lan e p features and es y. The th s wa llinator do thi e of po y d in e typ h ate o th examples: W llin ed t mmon po lat e co ts re om lan are e s How? p rs ar ive e re ation? at ow He f pollin n f t. pe o d e ac is ty uide how an tiv tr th will g al a at for nts ave. e n y t pla ny to h Z Z e lan ur ma w N th p yo ow e f t to its d h N o a is an o? e h w v n Wh p t o at de a H th ar ator: h r g ollin s o e of p at th ype in in T ll s po t f an o pl e r p u y o t y e h ce T la p to You don’t need a lot of space to ensure your garden is planted for successful pollination and attracts Landscapes the right pollinators. Bees, butterflies, moths, birds, lizards and even flies help to pollinate our native flowers. This enables our plants to reproduce and bear fruit. For native planting in your garden to be successful and to sustain itself into the future it is important for plants to be selected and For Life planted to ensure pollination is achieved. Each native plant needs to be planted in a different way depending on the Selecting and planting for poLlination type of pollinator that its flowers need.