Flower Investigation and Activities
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Selfing Can Facilitate Transitions Between Pollination Syndromes
vol. 191, no. 5 the american naturalist may 2018 Selfing Can Facilitate Transitions between Pollination Syndromes Carolyn A. Wessinger* and John K. Kelly Department of Ecology and Evolutionary Biology, University of Kansas, Lawrence, Kansas 66045 Submitted August 17, 2017; Accepted November 2, 2017; Electronically published March 14, 2018 Online enhancements: appendixes. Dryad data: http://dx.doi.org/10.5061/dryad.8hc64. fi abstract: Pollinator-mediated selection on plants can favor tran- (Herrera 1987). When pollen is limiting, pollinator ef ciency sitions to a new pollinator depending on the relative abundances and can determine fruit set per visit (Schemske and Horvitz efficiencies of pollinators present in the community. A frequently ob- 1984). Since pollinators differ in their receptiveness to floral served example is the transition from bee pollination to humming- signals and rewards as well as in how they interact with bird pollination. We present a population genetic model that examines flowers, pollinator-mediated selection has led to the wide- whether the ability to inbreed can influence evolutionary change in spread convergent evolution of pollination syndromes—sets fi traits that underlie pollinator attraction. We nd that a transition to of floral traits associated with certain types of pollinators a more efficient but less abundant pollinator is favored under a broad- ened set of ecological conditions if plants are capable of delayed selfing (Faegri and Van der Pijl 1979; Fenster et al. 2004; Harder rather than obligately outcrossing. Delayed selfing allows plants carry- and Johnson 2009). Pollinator communities vary over space ing an allele that attracts the novel pollinator to reproduce even when and time, leading to repeated evolutionary transitions in pol- this pollinator is rare, providing reproductive assurance. -

Pollination and Evolution of Plant and Insect Interaction JPP 2017; 6(3): 304-311 Received: 03-03-2017 Accepted: 04-04-2017 Showket a Dar, Gh
Journal of Pharmacognosy and Phytochemistry 2017; 6(3): 304-311 E-ISSN: 2278-4136 P-ISSN: 2349-8234 Pollination and evolution of plant and insect interaction JPP 2017; 6(3): 304-311 Received: 03-03-2017 Accepted: 04-04-2017 Showket A Dar, Gh. I Hassan, Bilal A Padder, Ab R Wani and Sajad H Showket A Dar Parey Sher-e-Kashmir University of Agricultural Science and Technology, Shalimar, Jammu Abstract and Kashmir-India Flowers exploit insects to achieve pollination; at the same time insects exploit flowers for food. Insects and flowers are a partnership. Each insect group has evolved different sets of mouthparts to exploit the Gh. I Hassan food that flowers provide. From the insects' point of view collecting nectar or pollen is rather like fitting Sher-e-Kashmir University of a key into a lock; the mouthparts of each species can only exploit flowers of a certain size and shape. Agricultural Science and This is why, to support insect diversity in our gardens, we need to plant a diversity of suitable flowers. It Technology, Shalimar, Jammu is definitely not a case of 'one size fits all'. While some insects are generalists and can exploit a wide and Kashmir-India range of flowers, others are specialists and are quite particular in their needs. In flowering plants, pollen grains germinate to form pollen tubes that transport male gametes (sperm cells) to the egg cell in the Bilal A Padder embryo sac during sexual reproduction. Pollen tube biology is complex, presenting parallels with axon Sher-e-Kashmir University of guidance and moving cell systems in animals. -

Pollination of Cultivated Plants in the Tropics 111 Rrun.-Co Lcfcnow!Cdgmencle
ISSN 1010-1365 0 AGRICULTURAL Pollination of SERVICES cultivated plants BUL IN in the tropics 118 Food and Agriculture Organization of the United Nations FAO 6-lina AGRICULTUTZ4U. ionof SERNES cultivated plans in tetropics Edited by David W. Roubik Smithsonian Tropical Research Institute Balboa, Panama Food and Agriculture Organization of the United Nations F'Ø Rome, 1995 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. M-11 ISBN 92-5-103659-4 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the copyright owner. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Publications Division, Food and Agriculture Organization of the United Nations, Viale delle Terme di Caracalla, 00100 Rome, Italy. FAO 1995 PlELi. uion are ted PlauAr David W. Roubilli (edita Footli-anal ISgt-iieulture Organization of the Untled Nations Contributors Marco Accorti Makhdzir Mardan Istituto Sperimentale per la Zoologia Agraria Universiti Pertanian Malaysia Cascine del Ricci° Malaysian Bee Research Development Team 50125 Firenze, Italy 43400 Serdang, Selangor, Malaysia Stephen L. Buchmann John K. S. Mbaya United States Department of Agriculture National Beekeeping Station Carl Hayden Bee Research Center P. -
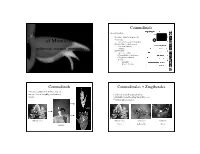
Diversity and Evolution of Monocots
Commelinids 4 main groups: Diversity and Evolution • Acorales - sister to all monocots • Alismatids of Monocots – inc. Aroids - jack in the pulpit • Lilioids (lilies, orchids, yams) – non-monophyletic . spiderworts, bananas, pineapples . – petaloid • Commelinids – Arecales – palms – Commelinales – spiderwort – Zingiberales –banana – Poales – pineapple – grasses & sedges Commelinids Commelinales + Zingiberales • theme: reduction of flower, loss of nectar, loss of zoophily, evolution of • 2 closely related tropical orders bracts • primarily nectar bearing but with losses • bracted inflorescences grass pickeral weed pickeral weed spiderwort heliconia nectar pollen only bracts rapatead bromeliad Commelinaceae - spiderwort Commelinaceae - spiderwort Family of small herbs with succulent stems, stems jointed; leaves sheathing. Family does not produce Inflorescence often bracted nectar, but showy flowers for insect pollen gathering. Rhoeo - Moses in a cradle Commelina erecta - Erect dayflower Tradescantia ohiensis - spiderwort Tradescantia ohiensis - spiderwort Commelinaceae - spiderwort Commelinaceae - spiderwort Flowers actinomorphic or • species rich in pantropics, CA 3 CO 3 A 6 G (3) zygomorphic especially Africa • floral diversity is enormous Commelina communis - day flower Tradescantia ohiensis - spiderwort Pontederiaceae - pickerel weed Pontederiaceae - pickerel weed Aquatic family of emergents or floaters. Pickerel weed has glossy heart-shaped leaves, Water hyacinth (Eichhornia) from superficially like Sagittaria but without net venation. -

Ornithophily in the Genus Salvia L. (Lamiaceae)
Ornithophily in the genus Salvia L. (Lamiaceae) Dissertation zur Erlangung des Grades „Doktor der Naturwissenschaften“ am Fachbereich Biologie der Johannes Gutenberg-Universität Mainz Petra Wester geb. in Linz/Rhein Mainz, 2007 Kapitel 2 dieser Arbeit wurde veröffentlicht beim Springer Verlag unter: Wester, P. & Claßen-Bockhoff, R. (2006): Hummingbird pollination in Salvia haenkei (Lamiaceae) lacking the typical lever mechanism. Plant Systematics and Evolution 257: 133-146. Kapitel 3 dieser Arbeit wurde veröffentlicht bei Elsevier unter: Wester, P. & Claßen- Bockhoff, R. (2006): Bird pollination in South African Salvia species. Flora 201: 396- 406. Kapitel 5 dieser Arbeit ist im Druck bei Oxford University Press (Annals of Botany) unter: Wester, P. & Claßen-Bockhoff, R. (2007): Floral diversity and pollen transfer mechanisms in bird-pollinated Salvia species. Meinen Eltern gewidmet Contents SUMMARY OF THE THESIS............................................................................................................................. 1 ZUSAMMENFASSUNG....................................................................................................................................... 2 1 GENERAL INTRODUCTION.......................................................................................................................... 3 2 HUMMINGBIRD POLLINATION IN SALVIA HAENKEI (LAMIACEAE) LACKING THE TYPICAL LEVER MECHANISM ..................................................................................................................................... -

Nectarivore Birds 3
Consideration of nectarivorous birds in wildlife risk assessments Agnes Schimera (1), Jan-Dieter Ludwigs (2), Oliver Koerner (1), Seamus Taylor (3) (1) ADAMA Deutschland GmbH; (2) RIFCON GmbH (3) ADAMA Agricultural Solutions Ltd. Contact: [email protected]; [email protected] 1 – Introduction In subtropical and tropical climate zones where crops exhibit a flowering phase before harvest, nectar-feeding birds (see table) may be attracted to crop flower nectar. We present points to consider on whether and how a nectarivorous avian scenario might be included in higher tier environmental risk assessment (ERA) for plant protection products (PPPs) and what data would be needed. Green-throated carib Eulampis holosericeus 2 - Nectarivore birds Bird Family Distribution Diet and hibiscus flower, Guadeloupe Hummingbirds New World 90% nectar, 10% small arthropods Nectar-feeding is widespread among birds, but almost no species (Trochilidae) Woodpeckers Worldwide Occasionally nectar, mainly insects, fruits consumes nectar exclusively. Most combine it with arthropods (Picidae) and other diet types for a mixed diet at least within parts of the Parrots Tropics, SE-Asia, Lories specialized brush-tipped tongue for year. (Psittacidae) Australasia nectar-feeding New Zealand Wrens New Zealand Supplementary (when insects scarce) Twelve families of birds contain more or less specialized (Acanthisittidae) Asities Madagascar Genus Neodrepanis primarily nectarivore, nectarivores (Bezzel and Prinzinger 1990). Of particular interest are (Philepittidae) otherwise supplementary three families: hummingbirds (Trochilidae), sunbirds (Nectariniidae) Australasian Tree- Australasia Insectivores, sometimes nectar and honeyeaters (Meliphagidae), which mainly drink nectar, and creepers (Climacteridae) Honeyeaters Australasia Specialized nectarivores, but also thereby collect pollen (Campbell and Lack 1985, Lovette and (Meliphagidae) invertebrates Fitzpatrick 2016). -

Plant-Hummingbird Interactions and Temporal Nectar Availability in a Restinga from Brazil
Anais da Academia Brasileira de Ciências (2015) 87(4): 2163-2175 (Annals of the Brazilian Academy of Sciences) Printed version ISSN 0001-3765 / Online version ISSN 1678-2690 http://dx.doi.org/10.1590/0001-3765201520140349 www.scielo.br/aabc Plant-hummingbird interactions and temporal nectar availability in a restinga from Brazil LORENA C.N. FONSECA1,5, JEFERSON VIZENTIN-BUGONI2,6, ANDRÉ R. RECH3 and MARIA ALICE S. ALVES4 1Programa de Pós-Graduação em Ecologia, Universidade Federal do Rio de Janeiro/ UFRJ, CCS, IB, Caixa Postal 68020, 21941-590 Rio de Janeiro, RJ, Brasil 2Programa de Pós-Graduação em Ecologia, Instituto de Biologia, Universidade Estadual de Campinas/ UNICAMP, Rua Monteiro Lobato, 970, Barão Geraldo, 13083-970 Campinas, SP, Brasil 3Instituto de Biologia, Universidade Federal de Uberlândia/UFU, Av. Pará, 1720, Campus Umuarama, 38405-320 Uberlândia, MG, Brasil 4Departamento de Ecologia, Instituto de Biologia Roberto Alcantara Gomes, Universidade do Estado do Rio de Janeiro/UERJ, Rua São Francisco Xavier, 524, Maracanã, 20550-011 Rio de Janeiro, RJ, Brasil 5Companhia Ambiental do Estado de São Paulo/CETESB, Av. Professor Frederico Hermann Jr, 345, Pinheiros, 05459-900 São Paulo, SP, Brasil 6Center for Macroecology, Evolution and Climate, Natural History Museum of Denmark, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen Ø, Denmark Manuscript received on July 10, 2014; accepted for publication on April 27, 2015 ABSTRACT Hummingbirds are the most important and specialized group of pollinating birds in the Neotropics and their interactions with plants are key components to many communities. In the present study we identified the assemblage of plants visited by hummingbirds and investigated the temporal availability of floral resources in an area of restinga, sandy plain coastal vegetation associated with the Atlantic forest, in Southeastern Brazil. -

Agents for Pollination by Durgeshwer Singh
AGENTS FOR POLLINATION Durgeshwer Singh Department of Botany Mahatma Gandhi Central University Agents for Pollination As the pollen is not capable of locomotion, pollination involves some agents for transfer of pollen grains especially in case of cross pollination. Cross Pollination Abiotic Agents Biotic Agents Entomophily Anemophily Ornithophily Hydrophily Cheiropteriphily Malacophily ABIOTIC AGENTS Anemophily (Pollination by air/ wind) Adaptation • Flowers- inconspicuous, usually not brightly coloured or scented • Petals are either small and green or absent • Male flowers are more numerous than female • Anther are versatile so that they swing freely by air currents • Pollen grains are smooth walled, relatively light, small and dry so they can be easily blown away by wind. • In grasses, pollen grains are relatively heavy and hence are not suitable for transport by wind. To overcome this problem, the male flowers are borne in the upper part of the inflorescence and the female in the lower part. • Examples; Most cereals and palms, Member of Salicaceae (Poplar, willow), Betulaceae (Alder, hazel, birch), Fagaceae (Oak, beech), Ulmaceae (Elm), Urticaceae (Urtica) etc. Hydrophily (Pollination by water) Hydrophilous flower are small and inconspicuous like anemophilous Hypo-hydrophily Epi-hydrophily • Pollination takes place completely under • Pollination of flower at the surface of water. water • More common • Example - Vallisneria • Pollination of flower below water level • Whole male flower break and float on the and is found in submerged plants like surface. Najas, Ceratophyllum and Zostera • Female flower are raised to the surface by • Aerenchyma present in anther- float a long spiral stalk. BIOTIC AGENTS Most important agent for pollination • Entomophily: pollination by Insects • Ornithophily: pollination by birds • Chiropteriphily: pollination by bats • Malacophily: pollination by slug and snail Entomophily (Pollination by insects) • Most frequent in Angiosperms. -

Historical Development of Ornithophily in the Western North American Flora
Proc. Nati. Acad. Sci. USA Vol. 91, pp. 10407-10411, October 1994 Evolution Historical development of ornithophily in the western North American flora (hummingbird po an system/evolution of mut /Arcto-Terlary floistic eemets/Madro-Tertlary flowisc eements) VERNE GRANT Department of Botany, University of Texas, Austin, TX 78713 Contributed by Verne Grant, July 18, 1994 ABSTRACT The 129 ornithophilous plant species in west- MATERIALS AND METHODS ern North America have floristic afites with one or the other of four geofloras: the Arcto-Tertiary flora (101 species), Western North America is defined for this study as the area Madro-Tertary flora (19 species), Madrean-Tethyan flora (8 from the Rocky Mountains to the Pacific coast, and from the species), and Neotropical flora (1 species). The last three floras Mexicanborderto southwestern Canadaand southern Alaska. have been in continuous contact with hmngblrds ince some The ornithophilous plant species in this area are listed in time early in the Tertiary, and ornithophily is old in this subset Table 1. The list includes 129 species in 39 genera and 18 of western ornithophilous plants. The Arcto-Tertiary flora had families. Hummingbird pollination records are available for no contact with hummingbirds in Eurasia or in its early history 43 of the species; the other species have inflorescence and in North America. Ornithophily is a new condition in Arcto- floral characters similar to those in the 43 documented Tertiary plant groups, dating from the firstdgncant contact species (1, 2). Some ornithophilous species may exist in of these plants with hummingbirds in the Eocene. Buidu of nature but be undetected and omitted from the list; ifso, their the hummingbird polination system in the Arcto-Tertary flora number would be small, and the listed species provide a good is expected to be gradual and stepwise for several reasons. -

Sexual Systems of Plants in a Brazilian Montane Forest
Floresta e Ambiente 2019; 26(Spec No 1): e20180394 https://doi.org/10.1590/2179-8087.039418 ISSN 2179-8087 (online) Original Article Conservation of Nature Sexual Systems of Plants in a Brazilian Montane Forest Monique Perini1 , Henrique Machado Dias2 , Sustanis Horn Kunz2 1Programa de Pós-graduação em Biologia Vegetal, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais – UFMG, Belo Horizonte/MG, Brasil 2Departamento de Ciências Florestais e da Madeira, Universidade Federal do Espírito Santo – UFES, Jerônimo Monteiro/ES, Brasil ABSTRACT In this study, vegetation reproduction has been investigated in order to understand aspects of speciation, structuring and composition of plant communities. Thus, we sought to characterize the frequency of sexual systems from species recorded in seed rain occurring in a tropical rainforest (Atlantic Forest) in Caparaó National Park, Espírito Santo State, Brazil. We collected the seed rain for twelve months, classified and recorded the species for: sexual system; pollination and dispersion syndrome; and fruit type. Then we measured the correlation between these attributes through correspondence analysis. Regarding sexual systems, 71% were hermaphrodites, 13% dioecious, and 11% monoecious. Hermaphrodites are best associated with pollination, dispersion and fruit types, represented by 65% of data variance. This study may contribute to elaborating management and conservation programs taking into account the interaction of plants with the local fauna. Keywords: seed rain, reproductive ecology, woody layer, Caparaó National Park, Atlantic Forest. Creative Commons License. All the contents of this journal, except where otherwise noted, is licensed under a Creative Commons Attribution License. 2/10 Perini M, Dias HM, Kunz SH Floresta e Ambiente 2019; 26(Spec No 1): e20180394 1. -

ORCHIDS and HUMMINGBIRDS: SEX in the FAST LANE Part 1 of Orchids and Their Pollinators CAROL SIEGEL
ORCHIDS AND HUMMINGBIRDS: SEX IN THE FAST LANE Part 1 of Orchids and Their Pollinators CAROL SIEGEL ART BULLY, ALL SWAGGER, hummingbirds are ing flowers locked together in a mutually beneficial tiny bundles of ego and attitude with no humili- dance. Pty or fear. The smallest warm-blooded avian crea- Hummingbirds (Trochilidae) are the predominant tures, they hover like a helicopter, consume energy like avian orchid pollinator. Birds are late-comers to the a jet plane, and glitter in the sunlight like a precious pollination game and only pollinate three percent of jewel. It is fitting that this most magnificent evolution- orchids. Nonetheless, with an estimated 35,000 orchid ary miracle should be a pollinator for the equally mag- species, there are probably hundreds and hundreds of nificent evolutionary miracle that is the orchid. orchids that rely on hummingbirds for pollination. Most orchids that are hummingbird- pollinated are from high- elevation ecosystems in the tropical New World where insects are rare or unable to operate because of the cold. They are particularly common in the Andean regions where hummingbirds reach their greatest diversity. Hummingbirds are found only in the Americas with at least 330 species from Alaska to the tip of South America. The greatest numbers are found in the tropics with fewer than 20 species normally found in the United States and Canada. Hummingbirds seem particularly attracted to many species of the genera Elleanthus, Cochlioda, and Comparettia. Some species of Masdevallia, Epidendrum, Encyclia, Cattleya, Sobralia, and Laelia have also adapted to hummingbirds. In addition, the highly-specialized little birds are attracted to certain species of Ada, Scaphyglottis (syn. -

3. the Importance of Bees in Nature
3. THE IMPORTANCE OF BEES IN NATURE BEES AS PART OF ECOSYSTEMS Pollinators strongly influence ecological relationships, ecosystem conservation and stability, genetic variation in the plant community, floral diversity, specialization and evolution. Bees play an important, but little recognized role in most terrestrial ecosystems where there is green vegetation cover for at least 3 to 4 months each year. In tropical forests, savannah woodlands, mangrove, and in temperate deciduous forests, many species of plants and animals would not survive if bees were missing. This is because the production of seeds, nuts, berries and fruits are highly dependent on insect pollination, and among the pollinating insects, bees are the major pollinators. In rain forests, especially in high mountain forests where it is too cold for most bees, other pollinators like bats and birds play a greater role in plant pollination. In farmed areas, bees are needed for the pollination of many cultivated crops (see Chapter 7), and for maintaining biodiversity in ‘islands’ of non-cultivated areas. The main role of bees in the different ecosystems is their pollination work. Other animal species are connected with bees: either because they eat the brood or honey, pollen or wax, because they are parasitic to the bees, or simply because they live within the bees nest. WHAT IS POLLINATION? Pollination is transfer of pollen from the anther (the male part of the flower) to the stigma (the female part of the flower). Some plants can pollinate themselves: in this case, the pollen passes from the anther to the stigma inside the same flower, and this is called self-pollination.