Pollination.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolutionary Ecology of Pollination and Reproduction of Tropical Plants
TROPICAL BIOLOGY AND CONSERVATION MANAGEMENT - Vol. V - Evolutionary Ecology af Pollination and Reproduction of Tropical Plants - M. Quesada, F. Rosas, Y. Herrerias-Diego, R. Aguliar, J.A. Lobo and G. Sanchez-Montoya EVOLUTIONARY ECOLOGY OF POLLINATION AND REPRODUCTION OF TROPICAL PLANTS M. Quesada and F. Rosas Centro de Investigaciones en Ecosistemas, Universidad Nacional Autónoma de México, México. Y. Herrerias-Diego Universidad Michoacana de San Nicolás de Hidalgo, Michoacán, México. R. Aguilar IMBIV - UNC - CONICET, C.C. 495,(5000) Córdoba, Argentina J.A. Lobo Escuela de Biología, Universidad de Costa Rica G. Sanchez-Montoya Centro de Investigaciones en Ecosistemas, Universidad Nacional Autónoma de México, México. Keywords: Pollination, tropical plants, diversity, mating systems, gender, conservation. Contents 1. Introduction 1.1. The Life Cycle of Angiosperms 1.2. Overview of Angiosperm Diversity 2. Degree of specificity of pollination system 3. Diversity of pollination systems 3.1. Beetle Pollination (Cantharophily) 3.2. Lepidoptera 3.2.1. Butterfly Pollination (Psychophily) 3.2.2. Moth Pollination (Phalaenophily) 3.3. Hymenoptera 3.3.1. Bee PollinationUNESCO (Melittophily) – EOLSS 3.3.2. Wasps 3.4. Fly Pollination (Myophily and Sapromyophily) 3.5. Bird Pollination (Ornitophily) 3.6. Bat PollinationSAMPLE (Chiropterophily) CHAPTERS 3.7. Pollination by No-Flying Mammals 3.8. Wind Pollination (Anemophily) 3.9. Water Pollination (Hydrophily) 4. Reproductive systems of angiosperms 4.1. Strategies that Reduce Selfing and/or Promote Cross-Pollination. 4.2. Self Incompatibility Systems 4.2.1. Incidence of Self Incompatibility in Tropical Forest 4.3. The Evolution of Separated Sexes from Hermaphroditism 4.3.1. From Distyly to Dioecy ©Encyclopedia Of. Life Support Systems (EOLSS) TROPICAL BIOLOGY AND CONSERVATION MANAGEMENT - Vol. -

Pollination and Evolution of Plant and Insect Interaction JPP 2017; 6(3): 304-311 Received: 03-03-2017 Accepted: 04-04-2017 Showket a Dar, Gh
Journal of Pharmacognosy and Phytochemistry 2017; 6(3): 304-311 E-ISSN: 2278-4136 P-ISSN: 2349-8234 Pollination and evolution of plant and insect interaction JPP 2017; 6(3): 304-311 Received: 03-03-2017 Accepted: 04-04-2017 Showket A Dar, Gh. I Hassan, Bilal A Padder, Ab R Wani and Sajad H Showket A Dar Parey Sher-e-Kashmir University of Agricultural Science and Technology, Shalimar, Jammu Abstract and Kashmir-India Flowers exploit insects to achieve pollination; at the same time insects exploit flowers for food. Insects and flowers are a partnership. Each insect group has evolved different sets of mouthparts to exploit the Gh. I Hassan food that flowers provide. From the insects' point of view collecting nectar or pollen is rather like fitting Sher-e-Kashmir University of a key into a lock; the mouthparts of each species can only exploit flowers of a certain size and shape. Agricultural Science and This is why, to support insect diversity in our gardens, we need to plant a diversity of suitable flowers. It Technology, Shalimar, Jammu is definitely not a case of 'one size fits all'. While some insects are generalists and can exploit a wide and Kashmir-India range of flowers, others are specialists and are quite particular in their needs. In flowering plants, pollen grains germinate to form pollen tubes that transport male gametes (sperm cells) to the egg cell in the Bilal A Padder embryo sac during sexual reproduction. Pollen tube biology is complex, presenting parallels with axon Sher-e-Kashmir University of guidance and moving cell systems in animals. -

Pollination of Cultivated Plants in the Tropics 111 Rrun.-Co Lcfcnow!Cdgmencle
ISSN 1010-1365 0 AGRICULTURAL Pollination of SERVICES cultivated plants BUL IN in the tropics 118 Food and Agriculture Organization of the United Nations FAO 6-lina AGRICULTUTZ4U. ionof SERNES cultivated plans in tetropics Edited by David W. Roubik Smithsonian Tropical Research Institute Balboa, Panama Food and Agriculture Organization of the United Nations F'Ø Rome, 1995 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. M-11 ISBN 92-5-103659-4 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the copyright owner. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Publications Division, Food and Agriculture Organization of the United Nations, Viale delle Terme di Caracalla, 00100 Rome, Italy. FAO 1995 PlELi. uion are ted PlauAr David W. Roubilli (edita Footli-anal ISgt-iieulture Organization of the Untled Nations Contributors Marco Accorti Makhdzir Mardan Istituto Sperimentale per la Zoologia Agraria Universiti Pertanian Malaysia Cascine del Ricci° Malaysian Bee Research Development Team 50125 Firenze, Italy 43400 Serdang, Selangor, Malaysia Stephen L. Buchmann John K. S. Mbaya United States Department of Agriculture National Beekeeping Station Carl Hayden Bee Research Center P. -

Lecture 4: ROLE of HONEY BEES in CROSS POLLINATION - THEIR EXPLOITATION - CASE STUDIES with SELECTED CROPS
Lecture 4: ROLE OF HONEY BEES IN CROSS POLLINATION - THEIR EXPLOITATION - CASE STUDIES WITH SELECTED CROPS For SEXUAL reproduction in flowering plants transfer of anther to stigma is essential - Pollination Self pollination Transfer to sligma of same plant No external agents are involved Cross pollination Transfer pollen from one plant to stigma of another plant External agents are involved External agents involved in pollination A. Abiotic agents a. Wind (Anemophily) Wind carries pollen from one plant to another Flowers are small, inconspicuous, unattractive Pollen are dry and light in weight Stigma feathery with large surface area eg: Maize, barley, wheat, sugarcane b. Water (Hydrophily) Water carries pollen from one plant to other B. Biotic agents Bird, bat and insects are important biotic agents Among insects honey bees play major role Honey bees and flowering plants have coevolved In insect pollinated plants, flowers are large, brightly colour, distinct fragrance, presence of nectar and sticky pollen True honeybees (Apis spp.) - Most valuable pollinators of commercial crop Qualities of honeybees which make them good pollinators 1. Body covered with hairs and have structural adaptation for carrying nectar and pollen. 2. Bees - Not injurious to plants 3. Adult and larva feed on nectar and pollen - Available in plenty 4. Superior pollinators - Since store pollen and nectar for future use 5. No diapause - Need pollen throughout year 6. Body size and probascis length - Suitable for many crops 7. Pollinate wide variety of crops 8. Forage -

Aerobiological Investigation and in Vitro Studies of Pollen Grains From
ORIGINAL ARTICLE Aerobiological Investigation and In Vitro Studies of Pollen Grains From 2 Dominant Avenue Trees in Kolkata, India J Mandal,1 I Roy,2 S Chatterjee,2 S Gupta-Bhattacharya1 1Division of Palynology and Environmental Biology, Department of Botany, Bose Institute, Kolkata, India 2Allergy Department, Institute of Child Health, Kolkata, India ■ Abstract Background: Peltophorum pterocarpum and Delonix regia are dominant avenue trees in the city of Kolkata in India. They are well adapted to the humid tropical climate and also grow commonly in different parts of the country. Their pollen grains are reported to be airborne. Objective: The aim of this study was to conduct an aerobiological survey in Kolkata to determine the concentration and seasonal periodicity of pollen grains from P pterocarpum and D regia and to analyze the meteorological factors responsible for their levels in the atmosphere. In addition, we analyzed the prevalence of sensitization due to these grains among patients with seasonal respiratory allergy. Methods: An aerobiological survey was conducted with a volumetric Burkard sampler from 2004 to 2006. Correlations between meteorological parameters and pollen grain concentrations were assessed by Spearman correlation test. The protein profi le of the pollen extracts was studied by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Finally, the allergenic potential of the pollen extracts was evaluated in patients with respiratory allergy by skin prick test, immunoglobulin (Ig) E enzyme-linked immunosorbent assay, and IgE immunoblotting. Results: P pterocarpum and D regia pollen grains occur from March to June and April to July, respectively. The pollen concentrations showed statistically signifi cant positive correlations with maximum temperature and wind speed. -
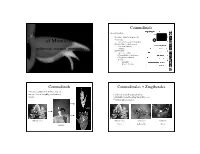
Diversity and Evolution of Monocots
Commelinids 4 main groups: Diversity and Evolution • Acorales - sister to all monocots • Alismatids of Monocots – inc. Aroids - jack in the pulpit • Lilioids (lilies, orchids, yams) – non-monophyletic . spiderworts, bananas, pineapples . – petaloid • Commelinids – Arecales – palms – Commelinales – spiderwort – Zingiberales –banana – Poales – pineapple – grasses & sedges Commelinids Commelinales + Zingiberales • theme: reduction of flower, loss of nectar, loss of zoophily, evolution of • 2 closely related tropical orders bracts • primarily nectar bearing but with losses • bracted inflorescences grass pickeral weed pickeral weed spiderwort heliconia nectar pollen only bracts rapatead bromeliad Commelinaceae - spiderwort Commelinaceae - spiderwort Family of small herbs with succulent stems, stems jointed; leaves sheathing. Family does not produce Inflorescence often bracted nectar, but showy flowers for insect pollen gathering. Rhoeo - Moses in a cradle Commelina erecta - Erect dayflower Tradescantia ohiensis - spiderwort Tradescantia ohiensis - spiderwort Commelinaceae - spiderwort Commelinaceae - spiderwort Flowers actinomorphic or • species rich in pantropics, CA 3 CO 3 A 6 G (3) zygomorphic especially Africa • floral diversity is enormous Commelina communis - day flower Tradescantia ohiensis - spiderwort Pontederiaceae - pickerel weed Pontederiaceae - pickerel weed Aquatic family of emergents or floaters. Pickerel weed has glossy heart-shaped leaves, Water hyacinth (Eichhornia) from superficially like Sagittaria but without net venation. -

Pollen Limitation and Flower Abortion in a Wind-Pollinated, Masting Tree
Notes Ecology, 96(2), 2015, pp. 587–593 Ó 2015 by the Ecological Society of America Pollen limitation and flower abortion in a wind-pollinated, masting tree 1,2,5 1,3 4 1 IAN S. PEARSE, WALTER D. KOENIG, KYLE A. FUNK, AND MARIO B. PESENDORFER 1Cornell Lab of Ornithology, 159 Sapsucker Woods Road, Ithaca, New York 14850 USA 2Illinois Natural History Survey, 1816 S. Oaks Street, Champaign, Illinois 61820 USA 3Department of Neurobiology and Behavior, Cornell University, Ithaca, New York 14853 USA 4School of Biological Sciences, University of Nebraska, Lincoln, Nebraska 68588 USA Abstract. Pollen limitation is a key assumption of theories that explain mast seeding, which is common among wind-pollinated and woody plants. In particular, the pollen coupling hypothesis and pollination Moran effect hypothesis assume pollen limitation as a factor that synchronizes seed crops across individuals. The existence of pollen limitation has not, however, been unambiguously demonstrated in wind-pollinated, masting trees. We conducted a two-year pollen supplementation experiment on a masting oak species, Quercus lobata. Supplemental pollen increased acorn set in one year but not in the other, supporting the importance of pollen coupling and pollination Moran effect models of mast seeding. We also tracked the fate of female flowers over five years and found that the vast majority of flowers were aborted for reasons unrelated to pollination, even in the presence of excess pollen. Pollen limitation can reduce annual seed set in a wind-pollinated tree, but factors other than pollen limitation cause the majority of flower abortion. Key words: anemophily; flower abortion; perennial plants; pollen; seed set; wind pollination. -

Pollination in Brazilian Syngonanthus (Eriocaulaceae) Species: Evidence for Entomophily Instead of Anemophily
Annals of Botany 96: 387–397, 2005 doi:10.1093/aob/mci191, available online at www.aob.oupjournals.org Pollination in Brazilian Syngonanthus (Eriocaulaceae) Species: Evidence for Entomophily Instead of Anemophily CARLIANNE O. C. RAMOS*, EDUARDO L. BORBA* and LI´GIA S. FUNCH Departamento de Cieˆncias Biolo´gicas, Laborato´rio de Taxonomia Vegetal, Universidade Estadual de Feira de Santana, Rodovia BR 116, km 03, Feira de Santana, BA, 44031-460, Brazil Received: 17 February 2005 Returned for revision: 1 April 2005 Accepted: 7 May 2005 Published electronically: 20 June 2005 Background and Aims The reproductive biology of Syngonanthus mucugensis and S. curralensis (Eriocaulaceae) was studied in areas of ‘campo rupestre’ vegetation in the Chapada Diamantina, north-eastern Brazil. These species are herbaceous and the individuals have a grouped distribution. Their leaves are united in a rosette, and their inflorescence is monoecious, of the capitulum type. The staminate and pistillate rings mature in a centripetal manner on the capitulum. Methods A field study was conducted, including observations concerning the morphology and biology of the flowers, fruit development, insect visits and anemophily, in both S. mucugensis and S. curralensis. Experimental pollinations were also carried out to study the mating systems of S. mucugensis. Key Results Both species flower from June to August. The staminate cycle lasts approx. 7 d, and the pistillate cycle from 3 to 4 d, with no temporal overlap between them on the same capitulum. The pollen viability of S. mucugensis was 88Á6 %, and 92Á5 % for S. curralensis. The inflorescences of both species demonstrated ultraviolet absorbance, and a sweet odour was detected during both the staminate and pistillate phases. -

Agents for Pollination by Durgeshwer Singh
AGENTS FOR POLLINATION Durgeshwer Singh Department of Botany Mahatma Gandhi Central University Agents for Pollination As the pollen is not capable of locomotion, pollination involves some agents for transfer of pollen grains especially in case of cross pollination. Cross Pollination Abiotic Agents Biotic Agents Entomophily Anemophily Ornithophily Hydrophily Cheiropteriphily Malacophily ABIOTIC AGENTS Anemophily (Pollination by air/ wind) Adaptation • Flowers- inconspicuous, usually not brightly coloured or scented • Petals are either small and green or absent • Male flowers are more numerous than female • Anther are versatile so that they swing freely by air currents • Pollen grains are smooth walled, relatively light, small and dry so they can be easily blown away by wind. • In grasses, pollen grains are relatively heavy and hence are not suitable for transport by wind. To overcome this problem, the male flowers are borne in the upper part of the inflorescence and the female in the lower part. • Examples; Most cereals and palms, Member of Salicaceae (Poplar, willow), Betulaceae (Alder, hazel, birch), Fagaceae (Oak, beech), Ulmaceae (Elm), Urticaceae (Urtica) etc. Hydrophily (Pollination by water) Hydrophilous flower are small and inconspicuous like anemophilous Hypo-hydrophily Epi-hydrophily • Pollination takes place completely under • Pollination of flower at the surface of water. water • More common • Example - Vallisneria • Pollination of flower below water level • Whole male flower break and float on the and is found in submerged plants like surface. Najas, Ceratophyllum and Zostera • Female flower are raised to the surface by • Aerenchyma present in anther- float a long spiral stalk. BIOTIC AGENTS Most important agent for pollination • Entomophily: pollination by Insects • Ornithophily: pollination by birds • Chiropteriphily: pollination by bats • Malacophily: pollination by slug and snail Entomophily (Pollination by insects) • Most frequent in Angiosperms. -

Influence of Recent Global Change on the Pollen Season in Europe
TECHNISCHE UNIVERSITÄT MÜNCHEN Fachgebiet für Ökoklimatologie Influence of recent global change on the pollen season in Europe Chiara Ziello Vollständiger Abdruck der von der Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt der Technischen Universität München zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigten Dissertation. Vorsitzender: Univ.-Prof. Dr. A. Göttlein Prüfer der Dissertation: 1. Univ.-Prof. Dr. A. Menzel 2. Univ.-Prof. D. P. Ankerst, Ph.D. Die Dissertation wurde am 11.06.2013 bei der Technischen Universität München ein- gereicht und durch die Fakultät Wissenschaftszentrum Weihenstephan für Ernäh- rung, Landnutzung und Umwelt am 22.07.2013 angenommen. Chiara Ziello: Influence of recent global change on the pollen season in Europe c 29.07.2013 supervisors: Univ.-Prof. Dr. A. Menzel Univ.-Prof. D. P. Ankerst, Ph.D. location: Freising Ohana means family. Family means nobody gets left behind, or forgotten. — Lilo & Stitch To my father, who taught me how quality is important to make a difference. ABSTRACT Background and objectives The reality of marked changes in current climate conditions is no longer under discussion. Climate change is now widely recognized as the major problem facing the planet, impacting animal and plant life, ecosystems, and the whole environment. Increasing risk of pollinosis (hay fever) is one of the most an- ticipated consequences of climate change on human health. In fact, a progressive global increase in the burden of allergic dis- eases has affected the industrialized world over the last half century. The clinical evidence reveals a general increase in both incidence and prevalence of respiratory diseases, such as aller- gic rhinitis (common hay fever) and asthma. -

Mellifera Mellifera
Journal of Apicultural Research 52(4): (2013) © IBRA 2013 DOI 10.3896/IBRA.1.52.4.12 REVIEW ARTICLE Standard methods for pollination research with Apis mellifera Keith S Delaplane1*, Arnon Dag2, Robert G Danka3, Breno M Freitas4, Lucas A Garibaldi5, 6 7 R Mark Goodwin and Jose I Hormaza 1Department of Entomology, University of Georgia, Athens, GA 30602, USA. 2Gilat Research Center, Agricultural Research Organization, Ministry of Agriculture, Mobile Post Negev 85280, Israel. 3Honey Bee Breeding, Genetics, and Physiology Research, 1157 Ben Hur Road, Baton Rouge, LA 70820, USA. 4Departamento de Zootecnia - CCA, Universidade Federal do Ceará, C.P. 12168, Fortaleza – CE, 60.021-970, Brazil. 5Sede Andina, Universidad Nacional de Río Negro (UNRN) and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Mitre 630, CP 8400, San Carlos de Bariloche, Río Negro, Argentina. 6The New Zealand Institute for Plant and Food Research Limited, Plant and Food Research Ruakura, Private Bag 3123, Hamilton 3240, New Zealand. 7Instituto de Hortofruticultura Subtropical y Mediterranea La Mayora (IHSM La Mayora-CSIC-UMA), 29750 Algarrobo-Costa, Málaga, Spain. Received 30 October 2012, accepted subject to revision 12 February 2013, accepted for publication 20 June 2013. *Corresponding author: Email: [email protected] Summary In this chapter we present a synthesis of recommendations for conducting field experiments with honey bees in the context of agricultural pollination. We begin with an overview of methods for determining the mating system requirements of plants and the efficacy of specific pollinators. We describe methods for evaluating the pollen-vectoring capacity of bees at the level of individuals or colonies and follow with methods for determining optimum colony field stocking densities. -

Sexual Systems of Plants in a Brazilian Montane Forest
Floresta e Ambiente 2019; 26(Spec No 1): e20180394 https://doi.org/10.1590/2179-8087.039418 ISSN 2179-8087 (online) Original Article Conservation of Nature Sexual Systems of Plants in a Brazilian Montane Forest Monique Perini1 , Henrique Machado Dias2 , Sustanis Horn Kunz2 1Programa de Pós-graduação em Biologia Vegetal, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais – UFMG, Belo Horizonte/MG, Brasil 2Departamento de Ciências Florestais e da Madeira, Universidade Federal do Espírito Santo – UFES, Jerônimo Monteiro/ES, Brasil ABSTRACT In this study, vegetation reproduction has been investigated in order to understand aspects of speciation, structuring and composition of plant communities. Thus, we sought to characterize the frequency of sexual systems from species recorded in seed rain occurring in a tropical rainforest (Atlantic Forest) in Caparaó National Park, Espírito Santo State, Brazil. We collected the seed rain for twelve months, classified and recorded the species for: sexual system; pollination and dispersion syndrome; and fruit type. Then we measured the correlation between these attributes through correspondence analysis. Regarding sexual systems, 71% were hermaphrodites, 13% dioecious, and 11% monoecious. Hermaphrodites are best associated with pollination, dispersion and fruit types, represented by 65% of data variance. This study may contribute to elaborating management and conservation programs taking into account the interaction of plants with the local fauna. Keywords: seed rain, reproductive ecology, woody layer, Caparaó National Park, Atlantic Forest. Creative Commons License. All the contents of this journal, except where otherwise noted, is licensed under a Creative Commons Attribution License. 2/10 Perini M, Dias HM, Kunz SH Floresta e Ambiente 2019; 26(Spec No 1): e20180394 1.