Functional Integration of Floral Plant Traits: Shape and Symmetry, Optical Signal, Reward and Reproduction in the Angiosperm Flower
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

High-Throughput Sequencing of Six Bamboo Chloroplast Genomes: Phylogenetic Implications for Temperate Woody Bamboos (Poaceae: Bambusoideae)
High-Throughput Sequencing of Six Bamboo Chloroplast Genomes: Phylogenetic Implications for Temperate Woody Bamboos (Poaceae: Bambusoideae) Yun-Jie Zhang1,2,3., Peng-Fei Ma1,2,3., De-Zhu Li1,2* 1 Key Laboratory of Biodiversity and Biogeography, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, People’s Republic of China, 2 Plant Germplasm and Genomics Center, Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, People’s Republic of China, 3 Graduate University of Chinese Academy of Sciences, Beijing, People’s Republic of China Abstract Background: Bambusoideae is the only subfamily that contains woody members in the grass family, Poaceae. In phylogenetic analyses, Bambusoideae, Pooideae and Ehrhartoideae formed the BEP clade, yet the internal relationships of this clade are controversial. The distinctive life history (infrequent flowering and predominance of asexual reproduction) of woody bamboos makes them an interesting but taxonomically difficult group. Phylogenetic analyses based on large DNA fragments could only provide a moderate resolution of woody bamboo relationships, although a robust phylogenetic tree is needed to elucidate their evolutionary history. Phylogenomics is an alternative choice for resolving difficult phylogenies. Methodology/Principal Findings: Here we present the complete nucleotide sequences of six woody bamboo chloroplast (cp) genomes using Illumina sequencing. These genomes are similar to those of other grasses and rather conservative in evolution. We constructed a phylogeny of Poaceae from 24 complete cp genomes including 21 grass species. Within the BEP clade, we found strong support for a sister relationship between Bambusoideae and Pooideae. In a substantial improvement over prior studies, all six nodes within Bambusoideae were supported with $0.95 posterior probability from Bayesian inference and 5/6 nodes resolved with 100% bootstrap support in maximum parsimony and maximum likelihood analyses. -

Apiaceae) - Beds, Old Cambs, Hunts, Northants and Peterborough
CHECKLIST OF UMBELLIFERS (APIACEAE) - BEDS, OLD CAMBS, HUNTS, NORTHANTS AND PETERBOROUGH Scientific name Common Name Beds old Cambs Hunts Northants and P'boro Aegopodium podagraria Ground-elder common common common common Aethusa cynapium Fool's Parsley common common common common Ammi majus Bullwort very rare rare very rare very rare Ammi visnaga Toothpick-plant very rare very rare Anethum graveolens Dill very rare rare very rare Angelica archangelica Garden Angelica very rare very rare Angelica sylvestris Wild Angelica common frequent frequent common Anthriscus caucalis Bur Chervil occasional frequent occasional occasional Anthriscus cerefolium Garden Chervil extinct extinct extinct very rare Anthriscus sylvestris Cow Parsley common common common common Apium graveolens Wild Celery rare occasional very rare native ssp. Apium inundatum Lesser Marshwort very rare or extinct very rare extinct very rare Apium nodiflorum Fool's Water-cress common common common common Astrantia major Astrantia extinct very rare Berula erecta Lesser Water-parsnip occasional frequent occasional occasional x Beruladium procurrens Fool's Water-cress x Lesser very rare Water-parsnip Bunium bulbocastanum Great Pignut occasional very rare Bupleurum rotundifolium Thorow-wax extinct extinct extinct extinct Bupleurum subovatum False Thorow-wax very rare very rare very rare Bupleurum tenuissimum Slender Hare's-ear very rare extinct very rare or extinct Carum carvi Caraway very rare very rare very rare extinct Chaerophyllum temulum Rough Chervil common common common common Cicuta virosa Cowbane extinct extinct Conium maculatum Hemlock common common common common Conopodium majus Pignut frequent occasional occasional frequent Coriandrum sativum Coriander rare occasional very rare very rare Daucus carota Wild Carrot common common common common Eryngium campestre Field Eryngo very rare, prob. -

Phylogeny and Classification of the Melastomataceae and Memecylaceae
Nord. J. Bot. - Section of tropical taxonomy Phylogeny and classification of the Melastomataceae and Memecy laceae Susanne S. Renner Renner, S. S. 1993. Phylogeny and classification of the Melastomataceae and Memecy- laceae. - Nord. J. Bot. 13: 519-540. Copenhagen. ISSN 0107-055X. A systematic analysis of the Melastomataceae, a pantropical family of about 4200- 4500 species in c. 166 genera, and their traditional allies, the Memecylaceae, with c. 430 species in six genera, suggests a phylogeny in which there are two major lineages in the Melastomataceae and a clearly distinct Memecylaceae. Melastomataceae have close affinities with Crypteroniaceae and Lythraceae, while Memecylaceae seem closer to Myrtaceae, all of which were considered as possible outgroups, but sister group relationships in this plexus could not be resolved. Based on an analysis of all morph- ological and anatomical characters useful for higher level grouping in the Melastoma- taceae and Memecylaceae a cladistic analysis of the evolutionary relationships of the tribes of the Melastomataceae was performed, employing part of the ingroup as outgroup. Using 7 of the 21 characters scored for all genera, the maximum parsimony program PAUP in an exhaustive search found four 8-step trees with a consistency index of 0.86. Because of the limited number of characters used and the uncertain monophyly of some of the tribes, however, all presented phylogenetic hypotheses are weak. A synapomorphy of the Memecylaceae is the presence of a dorsal terpenoid-producing connective gland, a synapomorphy of the Melastomataceae is the perfectly acrodro- mous leaf venation. Within the Melastomataceae, a basal monophyletic group consists of the Kibessioideae (Prernandra) characterized by fiber tracheids, radially and axially included phloem, and median-parietal placentation (placentas along the mid-veins of the locule walls). -

Siebold and Zuccarini's Type Specimens and Original Materials
J. Jpn. Bot. 89: 209–229 (2014) Siebold and Zuccarini’s Type Specimens and Original Materials from Japan, Part 4. Angiosperms. Dicotyledoneae 3 a, b c Shinobu AKIYAMA *, Gerard THIJSSE , Hans-Joachim ESSER d and Hideaki OHBA aDepartment of Botany, National Museum of Nature and Science, 4-1-1, Amakubo, Tsukuba, 305-0005 JAPAN; bNationaal Herbarium Nederland, Naturalis Biodiversity Center, P. O. Box 9514, THE NETHERLANDS; cBotanische Staatssammlung München, Menzinger Straße 67, D-80638 München, GERMANY; dDepartment of Botany, the University Museum, the University of Tokyo, 7-3-1, Hongo, Tokyo, 113-0033 JAPAN *Corresponding author: [email protected] (Accepted on March 1, 2014) Part 4 of the list of type specimens and original materials of the taxa described by Siebold and Zuccarini from Japan concerns the taxa belonging to Piperaceae to Hamamelidaceae, according to the 12th Engler system by Melchior. (Continued from J. Jpn. Bot. 89: 76–102, 2014) Key words: Japanese flora, lectotypification, Siebold collections, Zuccarini. This paper is the part 4 of the list of type Original material: In Japonia legt. Bürger, specimens and original materials of the taxa Herb. Zuccarinii (M0153560) [Category 2]. In described by Siebold and Zuccarini from Japonia legt. Bürger comct. d. Siebold, Herb. Japan (Akiyama et al. 2012, 2013, 2014), and Zuccarinii (M0153559) [Category 2b]. Japonia. treats the taxa belonging to Piperaceae to [Siebold s.n.] (MAK S1676) [Category 2]. Hamamelidaceae according to the 12th Engler system by Melchior. The method, especially the Chloranthaceae evaluation principles of category of the original Chloranthus Sw. materials, is the same as explained in part 1. -

Downloaded from Public
bioRxiv preprint doi: https://doi.org/10.1101/2020.10.16.341925; this version posted October 17, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Pre-meiotic, 21-nucleotide Reproductive PhasiRNAs Emerged in Seed Plants and Diversified in Flowering Plants Suresh Pokhrel1,2, Kun Huang3, Sébastien Bélanger1 , Jeffrey L. Caplan3, Elena M. Kramer4 and Blake C. Meyers1,2* 1 Donald Danforth Plant Science Center, Saint Louis, MO 63132 2 Division of Plant Sciences, University of Missouri-Columbia, Columbia, MO 65211 3 Bio-Imaging Center, Delaware Biotechnology Institute, University of Delaware, Newark, DE 19711, USA 4Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA, 02138 *Corresponding author: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/2020.10.16.341925; this version posted October 17, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Abstract Plant small RNAs (sRNAs) are important regulatory elements that fine-tune gene expression and maintain genome integrity by silencing transposons. They have critical roles in most pathways involved in plant growth and reproductive development. Reproductive organs of monocots produce abundant phased, small interfering RNAs (phasiRNAs). The 21-nt reproductive phasiRNAs triggered by miR2118 are highly enriched in pre-meiotic anthers, and have not been described in eudicots. -

Phylogeny and Subfamilial Classification of the Grasses (Poaceae) Author(S): Grass Phylogeny Working Group, Nigel P
Phylogeny and Subfamilial Classification of the Grasses (Poaceae) Author(s): Grass Phylogeny Working Group, Nigel P. Barker, Lynn G. Clark, Jerrold I. Davis, Melvin R. Duvall, Gerald F. Guala, Catherine Hsiao, Elizabeth A. Kellogg, H. Peter Linder Source: Annals of the Missouri Botanical Garden, Vol. 88, No. 3 (Summer, 2001), pp. 373-457 Published by: Missouri Botanical Garden Press Stable URL: http://www.jstor.org/stable/3298585 Accessed: 06/10/2008 11:05 Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/action/showPublisher?publisherCode=mobot. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit organization founded in 1995 to build trusted digital archives for scholarship. We work with the scholarly community to preserve their work and the materials they rely upon, and to build a common research platform that promotes the discovery and use of these resources. For more information about JSTOR, please contact [email protected]. -

Integrating Palaeontological and Molecular Data Uncovers Multiple
Integrating palaeontological and molecular data uncovers multiple ancient and recent dispersals in the pantropical Hamamelidaceae Xiaoguo Xiang, Kunli Xiang, Rosa del C. Ortiz, Florian Jabbour, Wei Wang To cite this version: Xiaoguo Xiang, Kunli Xiang, Rosa del C. Ortiz, Florian Jabbour, Wei Wang. Integrating palaeontolog- ical and molecular data uncovers multiple ancient and recent dispersals in the pantropical Hamamel- idaceae. Journal of Biogeography, Wiley, 2019, 46 (11), pp.2622-2631. 10.1111/jbi.13690. hal- 02612865 HAL Id: hal-02612865 https://hal.archives-ouvertes.fr/hal-02612865 Submitted on 19 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Integrating palaeontological and molecular data uncovers multiple ancient and recent dispersals in the pantropical Hamamelidaceae Xiaoguo Xiang1,2, Kunli Xiang1,3, Rosa Del C. Ortiz4, Florian Jabbour5, Wei Wang1,3 1State Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Beijing, China 2Jiangxi Province Key Laboratory of Watershed Ecosystem -
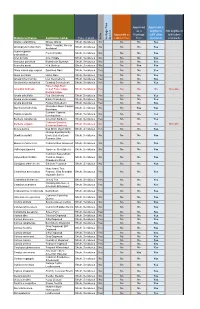
Botanical Name Common Name
Approved Approved & as a eligible to Not eligible to Approved as Frontage fulfill other fulfill other Type of plant a Street Tree Tree standards standards Heritage Tree Tree Heritage Species Botanical Name Common name Native Abelia x grandiflora Glossy Abelia Shrub, Deciduous No No No Yes White Forsytha; Korean Abeliophyllum distichum Shrub, Deciduous No No No Yes Abelialeaf Acanthropanax Fiveleaf Aralia Shrub, Deciduous No No No Yes sieboldianus Acer ginnala Amur Maple Shrub, Deciduous No No No Yes Aesculus parviflora Bottlebrush Buckeye Shrub, Deciduous No No No Yes Aesculus pavia Red Buckeye Shrub, Deciduous No No Yes Yes Alnus incana ssp. rugosa Speckled Alder Shrub, Deciduous Yes No No Yes Alnus serrulata Hazel Alder Shrub, Deciduous Yes No No Yes Amelanchier humilis Low Serviceberry Shrub, Deciduous Yes No No Yes Amelanchier stolonifera Running Serviceberry Shrub, Deciduous Yes No No Yes False Indigo Bush; Amorpha fruticosa Desert False Indigo; Shrub, Deciduous Yes No No No Not eligible Bastard Indigo Aronia arbutifolia Red Chokeberry Shrub, Deciduous Yes No No Yes Aronia melanocarpa Black Chokeberry Shrub, Deciduous Yes No No Yes Aronia prunifolia Purple Chokeberry Shrub, Deciduous Yes No No Yes Groundsel-Bush; Eastern Baccharis halimifolia Shrub, Deciduous No No Yes Yes Baccharis Summer Cypress; Bassia scoparia Shrub, Deciduous No No No Yes Burning-Bush Berberis canadensis American Barberry Shrub, Deciduous Yes No No Yes Common Barberry; Berberis vulgaris Shrub, Deciduous No No No No Not eligible European Barberry Betula pumila -

Number 3, Spring 1998 Director’S Letter
Planning and planting for a better world Friends of the JC Raulston Arboretum Newsletter Number 3, Spring 1998 Director’s Letter Spring greetings from the JC Raulston Arboretum! This garden- ing season is in full swing, and the Arboretum is the place to be. Emergence is the word! Flowers and foliage are emerging every- where. We had a magnificent late winter and early spring. The Cornus mas ‘Spring Glow’ located in the paradise garden was exquisite this year. The bright yellow flowers are bright and persistent, and the Students from a Wake Tech Community College Photography Class find exfoliating bark and attractive habit plenty to photograph on a February day in the Arboretum. make it a winner. It’s no wonder that JC was so excited about this done soon. Make sure you check of themselves than is expected to seedling selection from the field out many of the special gardens in keep things moving forward. I, for nursery. We are looking to propa- the Arboretum. Our volunteer one, am thankful for each and every gate numerous plants this spring in curators are busy planting and one of them. hopes of getting it into the trade. preparing those gardens for The magnolias were looking another season. Many thanks to all Lastly, when you visit the garden I fantastic until we had three days in our volunteers who work so very would challenge you to find the a row of temperatures in the low hard in the garden. It shows! Euscaphis japonicus. We had a twenties. There was plenty of Another reminder — from April to beautiful seven-foot specimen tree damage to open flowers, but the October, on Sunday’s at 2:00 p.m. -

Systematics and Relationships of Tryssophyton (Melastomataceae
A peer-reviewed open-access journal PhytoKeys 136: 1–21 (2019)Systematics and relationships of Tryssophyton (Melastomataceae) 1 doi: 10.3897/phytokeys.136.38558 RESEARCH ARTICLE http://phytokeys.pensoft.net Launched to accelerate biodiversity research Systematics and relationships of Tryssophyton (Melastomataceae), with a second species from the Pakaraima Mountains of Guyana Kenneth J. Wurdack1, Fabián A. Michelangeli2 1 Department of Botany, MRC-166 National Museum of Natural History, Smithsonian Institution, P.O. Box 37012, Washington, DC 20013-7012, USA 2 The New York Botanical Garden, 2900 Southern Blvd., Bronx, NY 10458, USA Corresponding author: Kenneth J. Wurdack ([email protected]) Academic editor: Ricardo Kriebel | Received 25 July 2019 | Accepted 30 October 2019 | Published 10 December 2019 Citation: Wurdack KJ, Michelangeli FA (2019) Systematics and relationships of Tryssophyton (Melastomataceae), with a second species from the Pakaraima Mountains of Guyana. PhytoKeys 136: 1–21. https://doi.org/10.3897/ phytokeys.136.38558 Abstract The systematics of Tryssophyton, herbs endemic to the Pakaraima Mountains of western Guyana, is re- viewed and Tryssophyton quadrifolius K.Wurdack & Michelang., sp. nov. from the summit of Kamakusa Mountain is described as the second species in the genus. The new species is distinguished from its closest relative, Tryssophyton merumense, by striking vegetative differences, including number of leaves per stem and leaf architecture. A phylogenetic analysis of sequence data from three plastid loci and Melastomata- ceae-wide taxon sampling is presented. The two species of Tryssophyton are recovered as monophyletic and associated with mostly Old World tribe Sonerileae. Fruit, seed and leaf morphology are described for the first time, biogeography is discussed and both species are illustrated. -

Identification of Giant Hogweed
Identification of Giant Hogweed Giant hogweed is an introduced plant species in New Brunswick. Currently, there are very few confirmed locations of this plant in the province. The majority are associated with a gardener who planted giant hogweed, which subsequently spread by unmanaged seed production. Giant hogweed is highly competitive due to its vigorous early-season growth, tolerance of full shade, and its ability to withstand flooding. Because of its large size and rapid growth, it can quickly dominate invaded areas and substantially reduce the amount of suitable habitat available for native plants and wildlife. In addition, this plant can cause potential human health issues. Giant hogweed sap contains toxins that, after exposure to sunlight, can cause significant dermatitis on sensitive individuals. Temporary blindness can also be caused by exposure of the eyes to the sap. Many plants within New Brunswick are similar in appearance to giant hogweed, most belonging to the Apiaceae or carrot family. Proper identification is essential to manage any plant species. Some of the most common similar species are listed below, with information to aid in proper identification. Giant Hogweed Giant hogweed (Heracleum mantegazzianum, Berce du Caucase) is a member of the Apiaceae family. It typically grows very tall, from 1.5 to 5 metres in height. Its leaves are shiny and large, with very coarse and serrated leaf edges, like a jagged saw edge. The stems are very hairy and bristly with purple spots or blotches throughout. When mature, the plant produces flowers that form a large umbrella shape, up to 1.5 metres in diameter. -

The Giant Hogweed Best Practice Manual
The giant hogweed best practice manual guidelines for the management and control of invasive weeds in Europe Booy, Olaf; Cock, Matthew; Eckstein, Lutz; Hansen, Steen Ole; Hattendorf, Jan; Hüls, Jörg; Jahodová, Sárka; Krinke, Lucás; Marovoková, Lanka; Müllerová, Jana; Nentwig, Wolfgang; Nielsen, Charlotte; Otte, Annette; Pergl, Jan; Perglová, Irena; Priekule, Ilze; Pusek, Petr; Ravn, Hans Peter; Thiele, Jan; Trybush, Sviatlana; Wittenberg, Rüdiger Publication date: 2005 Document version Publisher's PDF, also known as Version of record Citation for published version (APA): Booy, O., Cock, M., Eckstein, L., Hansen, S. O., Hattendorf, J., Hüls, J., Jahodová, S., Krinke, L., Marovoková, L., Müllerová, J., Nentwig, W., Nielsen, C., Otte, A., Pergl, J., Perglová, I., Priekule, I., Pusek, P., Ravn, H. P., Thiele, J., ... Wittenberg, R. (2005). The giant hogweed best practice manual: guidelines for the management and control of invasive weeds in Europe. Center for Skov, Landskab og Planlægning/Københavns Universitet. Download date: 28. sep.. 2021 The Giant Hogweed Best Practice Manual Guidelines for the management and control of an invasive weed in Europe manual_engelsk.pmd 45 14-07-2005, 14:20 About the publication Editors: Charlotte Nielsen, Hans Peter Ravn, Wolfgang Nentwig and Max Wade The project: Giant Alien Project (2002-2005) has been financed by the European Commission within the 5th Framework Pro- gramme, ’EESD – Energy, Environment and Sustainable Development’, contract no. EVK2-CT-2001-00128. Project partners are: Danish Centre for