Micrometre and Nanometre Scale Patterning of Binary Polymer Brushes, Supported Lipid Bilayers Cite This: Chem
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nanoscience and Nanotechnologies: Opportunities and Uncertainties
ISBN 0 85403 604 0 © The Royal Society 2004 Apart from any fair dealing for the purposes of research or private study, or criticism or review, as permitted under the UK Copyright, Designs and Patents Act (1998), no part of this publication may be reproduced, stored or transmitted in any form or by any means, without the prior permission in writing of the publisher, or, in the case of reprographic reproduction, in accordance with the terms of licences issued by the Copyright Licensing Agency in the UK, or in accordance with the terms of licenses issued by the appropriate reproduction rights organization outside the UK. Enquiries concerning reproduction outside the terms stated here should be sent to: Science Policy Section The Royal Society 6–9 Carlton House Terrace London SW1Y 5AG email [email protected] Typeset in Frutiger by the Royal Society Proof reading and production management by the Clyvedon Press, Cardiff, UK Printed by Latimer Trend Ltd, Plymouth, UK ii | July 2004 | Nanoscience and nanotechnologies The Royal Society & The Royal Academy of Engineering Nanoscience and nanotechnologies: opportunities and uncertainties Contents page Summary vii 1 Introduction 1 1.1 Hopes and concerns about nanoscience and nanotechnologies 1 1.2 Terms of reference and conduct of the study 2 1.3 Report overview 2 1.4 Next steps 3 2 What are nanoscience and nanotechnologies? 5 3 Science and applications 7 3.1 Introduction 7 3.2 Nanomaterials 7 3.2.1 Introduction to nanomaterials 7 3.2.2 Nanoscience in this area 8 3.2.3 Applications 10 3.3 Nanometrology -

UNEP Frontiers 2016 Report: Emerging Issues of Environmental Concern
www.unep.org United Nations Environment Programme P.O. Box 30552, Nairobi 00100, Kenya Tel: +254-(0)20-762 1234 Fax: +254-(0)20-762 3927 Email: [email protected] web: www.unep.org UNEP FRONTIERS 978-92-807-3553-6 DEW/1973/NA 2016 REPORT Emerging Issues of Environmental Concern 2014 © 2016 United Nations Environment Programme ISBN: 978-92-807-3553-6 Job Number: DEW/1973/NA Disclaimer This publication may be reproduced in whole or in part and in any form for educational or non-profit services without special permission from the copyright holder, provided acknowledgement of the source is made. UNEP would appreciate receiving a copy of any publication that uses this publication as a source. No use of this publication may be made for resale or any other commercial purpose whatsoever without prior permission in writing from the United Nations Environment Programme. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, DCPI, UNEP, P.O. Box 30552, Nairobi, 00100, Kenya. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of UNEP concerning the legal status of any country, territory or city or its authorities, or concerning the delimitation of its frontiers or boundaries. For general guidance on matters relating to the use of maps in publications please go to: http://www.un.org/Depts/Cartographic/english/htmain.htm Mention of a commercial company or product in this publication does not imply endorsement by the United Nations Environment Programme. -

It's a Nano World
IT’S A NANO WORLD Learning Goal • Nanometer-sized things are very small. Students can understand relative sizes of different small things • How Scientists can interact with small things. Understand Scientists and engineers have formed the interdisciplinary field of nanotechnology by investigating properties and manipulating matter at the nanoscale. • You can be a scientist DESIGNED FOR C H I L D R E N 5 - 8 Y E A R S O L D SO HOW SMALL IS NANO? ONE NANOMETRE IS A BILLIONTH O F A M E T R E Nanometre is a basic unit of measurement. “Nano” derives from the Greek word for midget, very small thing. If we divide a metre by 1 thousand we have a millimetre. One thousandth of a millimetre is a micron. A thousandth part of a micron is a nanometre. MACROSCALE OBJECTS 271 meters long. Humpback whales are A full-size soccer ball is Raindrops are around 0.25 about 14 meters long. 70 centimeters in diameter centimeters in diameter. MICROSCALE OBJECTS The diameter of Pollen, which human hairs ranges About 7 micrometers E. coli bacteria, found in fertilizes seed plants, from 50-100 across our intestines, are can be about 50 micrometers. around 2 micrometers micrometers in long. diameter. NANOSCALE OBJECTS The Ebola virus, The largest naturally- which causes a DNA molecules, which Water molecules are occurring atom is bleeding disease, is carry genetic code, are 0.278 nanometers wide. uranium, which has an around 80 around 2.5 nanometers atomic radius of 0.175 nanometers long. across. nanometers. TRY THIS! Mark your height on the wall chart. -

Orders of Magnitude (Length) - Wikipedia
03/08/2018 Orders of magnitude (length) - Wikipedia Orders of magnitude (length) The following are examples of orders of magnitude for different lengths. Contents Overview Detailed list Subatomic Atomic to cellular Cellular to human scale Human to astronomical scale Astronomical less than 10 yoctometres 10 yoctometres 100 yoctometres 1 zeptometre 10 zeptometres 100 zeptometres 1 attometre 10 attometres 100 attometres 1 femtometre 10 femtometres 100 femtometres 1 picometre 10 picometres 100 picometres 1 nanometre 10 nanometres 100 nanometres 1 micrometre 10 micrometres 100 micrometres 1 millimetre 1 centimetre 1 decimetre Conversions Wavelengths Human-defined scales and structures Nature Astronomical 1 metre Conversions https://en.wikipedia.org/wiki/Orders_of_magnitude_(length) 1/44 03/08/2018 Orders of magnitude (length) - Wikipedia Human-defined scales and structures Sports Nature Astronomical 1 decametre Conversions Human-defined scales and structures Sports Nature Astronomical 1 hectometre Conversions Human-defined scales and structures Sports Nature Astronomical 1 kilometre Conversions Human-defined scales and structures Geographical Astronomical 10 kilometres Conversions Sports Human-defined scales and structures Geographical Astronomical 100 kilometres Conversions Human-defined scales and structures Geographical Astronomical 1 megametre Conversions Human-defined scales and structures Sports Geographical Astronomical 10 megametres Conversions Human-defined scales and structures Geographical Astronomical 100 megametres 1 gigametre -

Glossary of Terms In
Glossary of Terms in Powder and Bulk Technology Prepared by Lyn Bates ISBN 978-0-946637-12-6 The British Materials Handling Board Foreward. Bulk solids play a vital role in human society, permeating almost all industrial activities and dominating many. Bulk technology embraces many disciplines, yet does not fall within the domain of a specific professional activity such as mechanical or chemical engineering. It has emerged comparatively recently as a coherent subject with tools for quantifying flow related properties and the behaviour of solids in handling and process plant. The lack of recognition of the subject as an established format with monumental industrial implications has impeded education in the subject. Minuscule coverage is offered within most university syllabuses. This situation is reinforced by the acceptance of empirical maturity in some industries and the paucity of quality textbooks available to address its enormous scope and range of application. Industrial performance therefore suffers. The British Materials Handling Board perceived the need for a Glossary of Terms in Particle Technology as an introductory tool for non-specialists, newcomers and students in this subject. Co-incidentally, a draft of a Glossary of Terms in Particulate Solids was in compilation. This concept originated as a project of the Working Part for the Mechanics of Particulate Solids, in support of a web site initiative of the European Federation of Chemical Engineers. The Working Party decided to confine the glossary on the EFCE web site to terms relating to bulk storage, flow of loose solids and relevant powder testing. Lyn Bates*, the UK industrial representative to the WPMPS leading this Glossary task force, decided to extend this work to cover broader aspects of particle and bulk technology and the BMHB arranged to publish this document as a contribution to the dissemination of information in this important field of industrial activity. -

13 the Rhetoric and Politics of Standardization: Measurements and Needs for Precision
13 The rhetoric and politics of standardization: Measurements and needs for precision Important then to escape the pitfalls of any arbitrariness in understanding what we are doing in counting units of length, weight, or any measurements of areas of space, we need to understand what constitutes the unit of measurement in order to use it practically in what we do when we use measuring tools. Measurements such as sums of units of length, area, of volume, angles, weight, and even sums of units of time are countable. In turn we use these measurements to relate to other things that we also define as being countable. How we relate to those countable applications depends upon the measuring tools we create. And what constitutes a unit of measurement is established with these measuring tools within the legal frameworks of what might be considered political power or law, whether laws of the tribe or the sovereign power of a nation. As experts we make political demands that become commands of those that have the political power to standardize measurements in manufacturing, construction, trade, and commerce. They create the law that underpins the technical definitional agreements that are made about measurements by those different social institutional agreements made between social corporate structures and government agencies. Measurements are always made then within certain social and political frameworks. The definitions of measurable units are usually initiated within the jurisdictions of technological and scientific institutions which with their persuasive power are able to eventually reach their legitimized agreements about standards, and they gain their approvable within law using enforceable legal contracts. -
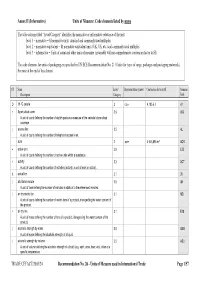
Units of Measure Used in International Trade Page 1/57 Annex II (Informative) Units of Measure: Code Elements Listed by Name
Annex II (Informative) Units of Measure: Code elements listed by name The table column titled “Level/Category” identifies the normative or informative relevance of the unit: level 1 – normative = SI normative units, standard and commonly used multiples level 2 – normative equivalent = SI normative equivalent units (UK, US, etc.) and commonly used multiples level 3 – informative = Units of count and other units of measure (invariably with no comprehensive conversion factor to SI) The code elements for units of packaging are specified in UN/ECE Recommendation No. 21 (Codes for types of cargo, packages and packaging materials). See note at the end of this Annex). ST Name Level/ Representation symbol Conversion factor to SI Common Description Category Code D 15 °C calorie 2 cal₁₅ 4,185 5 J A1 + 8-part cloud cover 3.9 A59 A unit of count defining the number of eighth-parts as a measure of the celestial dome cloud coverage. | access line 3.5 AL A unit of count defining the number of telephone access lines. acre 2 acre 4 046,856 m² ACR + active unit 3.9 E25 A unit of count defining the number of active units within a substance. + activity 3.2 ACT A unit of count defining the number of activities (activity: a unit of work or action). X actual ton 3.1 26 | additional minute 3.5 AH A unit of time defining the number of minutes in addition to the referenced minutes. | air dry metric ton 3.1 MD A unit of count defining the number of metric tons of a product, disregarding the water content of the product. -

Engilab Units 2018 V2.2
EngiLab Units 2021 v3.1 (v3.1.7864) User Manual www.engilab.com This page intentionally left blank. EngiLab Units 2021 v3.1 User Manual (c) 2021 EngiLab PC All rights reserved. No parts of this work may be reproduced in any form or by any means - graphic, electronic, or mechanical, including photocopying, recording, taping, or information storage and retrieval systems - without the written permission of the publisher. Products that are referred to in this document may be either trademarks and/or registered trademarks of the respective owners. The publisher and the author make no claim to these trademarks. While every precaution has been taken in the preparation of this document, the publisher and the author assume no responsibility for errors or omissions, or for damages resulting from the use of information contained in this document or from the use of programs and source code that may accompany it. In no event shall the publisher and the author be liable for any loss of profit or any other commercial damage caused or alleged to have been caused directly or indirectly by this document. "There are two possible outcomes: if the result confirms the Publisher hypothesis, then you've made a measurement. If the result is EngiLab PC contrary to the hypothesis, then you've made a discovery." Document type Enrico Fermi User Manual Program name EngiLab Units Program version v3.1.7864 Document version v1.0 Document release date July 13, 2021 This page intentionally left blank. Table of Contents V Table of Contents Chapter 1 Introduction to EngiLab Units 1 1 Overview .................................................................................................................................. -

SI Units in Geotechnical Engineering
R. D. Holtz ~ SI Units in Geotechnical Engineering REFERENCE: Holtz, R. D., "SI Units in Geoteehnleal Engineering," tinental European engineers. At least they tried to keep the distinc- Geotechnical Testing Journal, GTJODJ, Vol. 3, No. 2, June 1980, pp. tion between mass and force by calling the kilogram-force a "kilo- 73-79. pond" (kp). ABSTRACT: A brief description is presented of the International A modernized version of the metric system has been developing System of Units (SI) as it might be applied to geotechnical engineering. over the past 30 years. The system is known as SI, which stands for Base as well as derived SI units that are of interest to geotechnical le Syst~me International d'Unitds (The International System of engineers are described in detail, and conversion factors for units in Units). It is described in detail in ASTM E 380, the Standard for common usage are given. A few examples of conversions are also Metric Practice, available in the back of every part of the Annual presented. Book of ASTM Standards. The system may soon become the KEY WORDS: units of measurement, metric system, symbols common system in the United States and the few other countries still using Imperial or British Engineering units. In fact, Great Within the scientific and engineering community, there has Britain itself converted completely to SI in 1972, and Australia, always been some confusion as to the proper system of units for Canada, and New Zealand are presently well along the way to physical measurements and quantities. Many schemes have been conversion. -

Metrication Is SUCCESSFUL
Metrication is SUCCESSFUL Metrication is SUCCESSFUL because it is: Simple The modern metric system, formally known as the International System of Units (SI), is the simplest and easiest-to-use system of measurement ever devised. In fact the metric system is the only system of measurement ever devised. All previous measuring methods were just a hodge-podge of randomly generated local measures. Unique The metric system is unique. Never before, has there been a method of measurement that has all the positive benefits as SI. Coherent The metric system uses the same decimal nature as our number system, and it uses the same mathematical rules and symbols that we use for the mathematic of numbers Capable The metric system is capable of measuring anything in any trade, profession, or other human activity. The metric system has no limitations. For example, you might measure the distance from here to the door in metres, the distance from between your home and your work in kilometres, the width of your little finger nail in millimetres, the diameter of the hairs on your head in micrometres, and the size of one of your cells in nanometres. Why stop there? With the SI prefixes, there is more than enough flexibility to measure from the size of sub-atomic particles – the diameter of an electron is about 6 femtometres – to the size of the whole Universe – the diameter of the Universe, as observed by the world's best telescopes, is about 250 yottametres. Equitable The key argument for using the metric system is that it is fair to all concerned. -

Probing the Electromagnetic Field of a 15-Nanometre Hotspot by Single Molecule Imaging
LETTER doi:10.1038/nature09698 Probing the electromagnetic field of a 15-nanometre hotspot by single molecule imaging Hu Cang1,2, Anna Labno2,3, Changgui Lu2, Xiaobo Yin1,2, Ming Liu2, Christopher Gladden2, Yongmin Liu2 & Xiang Zhang1,2 When light illuminates a rough metallic surface, hotspots can techniques, together with structural illumination microscopy and sti- appear, where the light is concentrated on the nanometre scale, mulated emission depletion microscopy, allow optical resolution producing an intense electromagnetic field. This phenomenon, beyond the diffraction limit in biological samples (for a review, see called the surface enhancement effect1,2, has a broad range of ref. 22). As photo-switching would be infeasible for investigating local potential applications, such as the detection of weak chemical sig- field, we use the Brownian motion of single dye molecules20,21,23 in a nals. Hotspots are believed to be associated with localized electro- solution to let the dyes scan the surface of single hotspots in a stochastic magnetic modes3,4, caused by the randomness of the surface manner, one molecule at a time. texture. Probing the electromagnetic field of the hotspots would Figure 1a illustrates the principle of this new technique. Here, a offer much insight towards uncovering the mechanism generating sample is submerged in a solution of fluorescent dye. The chamber the enhancement; however, it requires a spatial resolution of containing the sample is mounted on a total internal reflection (TIRF) 1–2 nm, which has been a long-standing challenge in optics. The set-up. As the diffusion of the dye molecules is much faster than the resolution of an optical microscope is limited to about half the image acquisition time (a 1-nm-diameter sphere diffuses through a wavelength of the incident light, approximately 200–300 nm. -

Good Practice Guide No. 118 a Beginner's Guide to Measurement
Good Practice Guide No. 118 A Beginner’s Guide to Measurement National Physical Laboratory Teddington, Middlesex, United Kingdom, TW11 0LW For more information, or for help with measurement problems, please visit: www.npl.co.uk ISSN: 1368-6550 © Queen’s Printer and Controller of HMSO, 2010. NPL wishes to acknowledge the support of NMO, LGC and NEL in the production of this guide. i A Beginner’s Guide to Measurement Version 3 Mike Goldsmith This Beginner’s Guide explains the fundamental concepts and basic facts about measurement, and in particular accurate measurement. It includes brief accounts of the role of measurement in modern and historical societies and explains the SI system, its base units and their relation to other units. The various organisations involved in measurement are introduced and their roles in linking all measurements to the SI base units through traceability chains explained. It includes general guidance about practical issues that affect the making of measurements, gives the meanings of key measurement terms, and explains the significance of such fundamental concepts as measurement traceability and calibration. ii Messen ist Wissen (Measurement is knowledge) Georg Simon Ohm (1789-1854) iii Foreword This beginner’s guide is intended for people with little or no experience of making accurate measurements but who wish to find out more about them, either because they intend to make or use accurate measurements themselves or to work with those who do. It may be of use to those starting careers in metrology or engineering, and to managers, research scientists, teachers, university students and those involved in the sale or marketing of measuring instruments.