Glossary of Terms In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thermodynamics Notes
Thermodynamics Notes Steven K. Krueger Department of Atmospheric Sciences, University of Utah August 2020 Contents 1 Introduction 1 1.1 What is thermodynamics? . .1 1.2 The atmosphere . .1 2 The Equation of State 1 2.1 State variables . .1 2.2 Charles' Law and absolute temperature . .2 2.3 Boyle's Law . .3 2.4 Equation of state of an ideal gas . .3 2.5 Mixtures of gases . .4 2.6 Ideal gas law: molecular viewpoint . .6 3 Conservation of Energy 8 3.1 Conservation of energy in mechanics . .8 3.2 Conservation of energy: A system of point masses . .8 3.3 Kinetic energy exchange in molecular collisions . .9 3.4 Working and Heating . .9 4 The Principles of Thermodynamics 11 4.1 Conservation of energy and the first law of thermodynamics . 11 4.1.1 Conservation of energy . 11 4.1.2 The first law of thermodynamics . 11 4.1.3 Work . 12 4.1.4 Energy transferred by heating . 13 4.2 Quantity of energy transferred by heating . 14 4.3 The first law of thermodynamics for an ideal gas . 15 4.4 Applications of the first law . 16 4.4.1 Isothermal process . 16 4.4.2 Isobaric process . 17 4.4.3 Isosteric process . 18 4.5 Adiabatic processes . 18 5 The Thermodynamics of Water Vapor and Moist Air 21 5.1 Thermal properties of water substance . 21 5.2 Equation of state of moist air . 21 5.3 Mixing ratio . 22 5.4 Moisture variables . 22 5.5 Changes of phase and latent heats . -

Carbon – Science and Technology
© Applied Science Innovations Pvt. Ltd., India Carbon – Sci. Tech. 1 (2010) 139 - 143 Carbon – Science and Technology ASI ISSN 0974 – 0546 http://www.applied-science-innovations.com ARTICLE Received :29/03/2010, Accepted :02/09/2010 ----------------------------------------------------------------------------------------------------------------------------- Ablation morphologies of different types of carbon in carbon/carbon composites Jian Yin, Hongbo Zhang, Xiang Xiong, Jinlv Zuo State Key Laboratory of Powder Metallurgy, Central South University, Lushan South Road, Changsha, China. --------------------------------------------------------------------------------------------------------------------------------------- 1. Introduction : Carbon/carbon (C/C) composites the ablation morphology and formation mechanism of combine good mechanical properties and designable each types of carbon. capabilities of composites and excellent ultrahigh temperature properties of carbon materials. They have In this study, ablation morphologies of resin-based low densities, high specific strength, good thermal carbon, carbon fibers, pyrolytic carbon with smooth stability, high thermal conductivity, low thermal laminar structure and rough laminar structure has been expansion coefficient and excellent ablation properties investigated in detail and their formation mechanisms [1]. As C/C composites show excellent characteristics were discussed. in both structural design and functional application, 2 Experimental : they have become one of the most competitive high temperature materials widely used in aviation and 2.1 Preparation of C/C composites : Bulk needled polyacrylonitrile (PAN) carbon fiber felts spacecraft industry [2 - 4]. In particular, they are were used as reinforcements. Three kinds of C/C considered to be the most suitable materials for solid composites, labeled as sample A, B and C, were rocket motor nozzles. prepared. Sample A is a C/C composite mainly with C/C composites are composed of carbon fibers and smooth laminar pyrolytic carbon, sample B is a C/C carbon matrices. -

Equations of State and Thermodynamics of Solids Using Empirical Corrections in the Quasiharmonic Approximation
PHYSICAL REVIEW B 84, 184103 (2011) Equations of state and thermodynamics of solids using empirical corrections in the quasiharmonic approximation A. Otero-de-la-Roza* and V´ıctor Luana˜ † Departamento de Qu´ımica F´ısica y Anal´ıtica, Facultad de Qu´ımica, Universidad de Oviedo, ES-33006 Oviedo, Spain (Received 24 May 2011; revised manuscript received 8 October 2011; published 11 November 2011) Current state-of-the-art thermodynamic calculations using approximate density functionals in the quasi- harmonic approximation (QHA) suffer from systematic errors in the prediction of the equation of state and thermodynamic properties of a solid. In this paper, we propose three simple and theoretically sound empirical corrections to the static energy that use one, or at most two, easily accessible experimental parameters: the room-temperature volume and bulk modulus. Coupled with an appropriate numerical fitting technique, we show that experimental results for three model systems (MgO, fcc Al, and diamond) can be reproduced to a very high accuracy in wide ranges of pressure and temperature. In the best available combination of functional and empirical correction, the predictive power of the DFT + QHA approach is restored. The calculation of the volume-dependent phonon density of states required by QHA can be too expensive, and we have explored simplified thermal models in several phases of Fe. The empirical correction works as expected, but the approximate nature of the simplified thermal model limits significantly the range of validity of the results. DOI: 10.1103/PhysRevB.84.184103 PACS number(s): 64.10.+h, 65.40.−b, 63.20.−e, 71.15.Nc I. -

VISCOSITY of a GAS -Dr S P Singh Department of Chemistry, a N College, Patna
Lecture Note on VISCOSITY OF A GAS -Dr S P Singh Department of Chemistry, A N College, Patna A sketchy summary of the main points Viscosity of gases, relation between mean free path and coefficient of viscosity, temperature and pressure dependence of viscosity, calculation of collision diameter from the coefficient of viscosity Viscosity is the property of a fluid which implies resistance to flow. Viscosity arises from jump of molecules from one layer to another in case of a gas. There is a transfer of momentum of molecules from faster layer to slower layer or vice-versa. Let us consider a gas having laminar flow over a horizontal surface OX with a velocity smaller than the thermal velocity of the molecule. The velocity of the gaseous layer in contact with the surface is zero which goes on increasing upon increasing the distance from OX towards OY (the direction perpendicular to OX) at a uniform rate . Suppose a layer ‘B’ of the gas is at a certain distance from the fixed surface OX having velocity ‘v’. Two layers ‘A’ and ‘C’ above and below are taken into consideration at a distance ‘l’ (mean free path of the gaseous molecules) so that the molecules moving vertically up and down can’t collide while moving between the two layers. Thus, the velocity of a gas in the layer ‘A’ ---------- (i) = + Likely, the velocity of the gas in the layer ‘C’ ---------- (ii) The gaseous molecules are moving in all directions due= to −thermal velocity; therefore, it may be supposed that of the gaseous molecules are moving along the three Cartesian coordinates each. -

Identifying the Elastic Isotropy of Architectured Materials Based On
Identifying the elastic isotropy of architectured materials based on deep learning method Anran Wei a, Jie Xiong b, Weidong Yang c, Fenglin Guo a, d, * a Department of Engineering Mechanics, School of Naval Architecture, Ocean and Civil Engineering, Shanghai Jiao Tong University, Shanghai 200240, China b Department of Mechanical Engineering, The Hong Kong Polytechnic University, Kowloon, Hong Kong, China c School of Aerospace Engineering and Applied Mechanics, Tongji University, Shanghai 200092, China d State Key Laboratory of Ocean Engineering, Shanghai Jiao Tong University, Shanghai 200240, China * Corresponding author, E-mail: [email protected] Abstract: With the achievement on the additive manufacturing, the mechanical properties of architectured materials can be precisely designed by tailoring microstructures. As one of the primary design objectives, the elastic isotropy is of great significance for many engineering applications. However, the prevailing experimental and numerical methods are normally too costly and time-consuming to determine the elastic isotropy of architectured materials with tens of thousands of possible microstructures in design space. The quick mechanical characterization is thus desired for the advanced design of architectured materials. Here, a deep learning-based approach is developed as a portable and efficient tool to identify the elastic isotropy of architectured materials directly from the images of their representative microstructures with arbitrary component distributions. The measure of elastic isotropy for architectured materials is derived firstly in this paper to construct a database with associated images of microstructures. Then a convolutional neural network is trained with the database. It is found that the convolutional neural network shows good performance on the isotropy identification. -
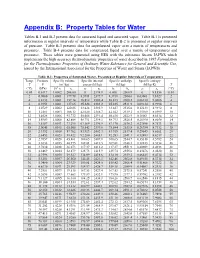
Appendix B: Property Tables for Water
Appendix B: Property Tables for Water Tables B-1 and B-2 present data for saturated liquid and saturated vapor. Table B-1 is presented information at regular intervals of temperature while Table B-2 is presented at regular intervals of pressure. Table B-3 presents data for superheated vapor over a matrix of temperatures and pressures. Table B-4 presents data for compressed liquid over a matrix of temperatures and pressures. These tables were generated using EES with the substance Steam_IAPWS which implements the high accuracy thermodynamic properties of water described in 1995 Formulation for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use, issued by the International Associated for the Properties of Water and Steam (IAPWS). Table B-1: Properties of Saturated Water, Presented at Regular Intervals of Temperature Temp. Pressure Specific volume Specific internal Specific enthalpy Specific entropy T P (m3/kg) energy (kJ/kg) (kJ/kg) (kJ/kg-K) T 3 (°C) (kPa) 10 vf vg uf ug hf hg sf sg (°C) 0.01 0.6117 1.0002 206.00 0 2374.9 0.000 2500.9 0 9.1556 0.01 2 0.7060 1.0001 179.78 8.3911 2377.7 8.3918 2504.6 0.03061 9.1027 2 4 0.8135 1.0001 157.14 16.812 2380.4 16.813 2508.2 0.06110 9.0506 4 6 0.9353 1.0001 137.65 25.224 2383.2 25.225 2511.9 0.09134 8.9994 6 8 1.0729 1.0002 120.85 33.626 2385.9 33.627 2515.6 0.12133 8.9492 8 10 1.2281 1.0003 106.32 42.020 2388.7 42.022 2519.2 0.15109 8.8999 10 12 1.4028 1.0006 93.732 50.408 2391.4 50.410 2522.9 0.18061 8.8514 12 14 1.5989 1.0008 82.804 58.791 2394.1 58.793 2526.5 -

States of Matter Lesson
National Aeronautics and Space Administration STATES OF MATTER NASA SUMMER OF INNOVATION LESSON DESCRIPTION UNIT This lesson explores the states of matter Physical Science—States of Matter and their properties. GRADE LEVELS OBJECTIVES 4 – 6 Students will CONNECTION TO CURRICULUM • Simulate the movement of atoms and molecules in solids, liquids, Science and gases TEACHER PREPARATION TIME • Demonstrate the properties of 2 hours liquids including density and buoyancy LESSON TIME NEEDED • Investigate how the density of a 4 hours Complexity: Moderate solid behaves in varying densities of liquids • Construct a rocket powered by pressurized gas created from a chemical reaction between a solid and a liquid NATIONAL STANDARDS National Science Education Standards (NSTA) Science and Technology • Abilities of technological design • Understanding science and technology Physical Science • Position and movement of objects • Properties and changes in properties of matter • Transfer of energy MANAGEMENT For the first activity you may need to enhance prior knowledge about matter and energy from a supplemental handout called “Diagramming Atoms and Molecules in Motion.” At the middle school level, this information about the invisible world of the atom is often presented as a story which we ask them to accept without much ready evidence. Since so many middle school students have not had science experience at the concrete operational level, they are poorly equipped to work at an abstract level. However, in this activity students can begin to see evidence that supports the abstract information you are sharing with them. They can take notes on the first two descriptions as you present on the overhead. Emphasize the spacing of the particles, rather than the number. -

Nanoscience and Nanotechnologies: Opportunities and Uncertainties
ISBN 0 85403 604 0 © The Royal Society 2004 Apart from any fair dealing for the purposes of research or private study, or criticism or review, as permitted under the UK Copyright, Designs and Patents Act (1998), no part of this publication may be reproduced, stored or transmitted in any form or by any means, without the prior permission in writing of the publisher, or, in the case of reprographic reproduction, in accordance with the terms of licences issued by the Copyright Licensing Agency in the UK, or in accordance with the terms of licenses issued by the appropriate reproduction rights organization outside the UK. Enquiries concerning reproduction outside the terms stated here should be sent to: Science Policy Section The Royal Society 6–9 Carlton House Terrace London SW1Y 5AG email [email protected] Typeset in Frutiger by the Royal Society Proof reading and production management by the Clyvedon Press, Cardiff, UK Printed by Latimer Trend Ltd, Plymouth, UK ii | July 2004 | Nanoscience and nanotechnologies The Royal Society & The Royal Academy of Engineering Nanoscience and nanotechnologies: opportunities and uncertainties Contents page Summary vii 1 Introduction 1 1.1 Hopes and concerns about nanoscience and nanotechnologies 1 1.2 Terms of reference and conduct of the study 2 1.3 Report overview 2 1.4 Next steps 3 2 What are nanoscience and nanotechnologies? 5 3 Science and applications 7 3.1 Introduction 7 3.2 Nanomaterials 7 3.2.1 Introduction to nanomaterials 7 3.2.2 Nanoscience in this area 8 3.2.3 Applications 10 3.3 Nanometrology -

Key Elements of X-Ray CT Physics Part 2: X-Ray Interactions
Key Elements of X-ray CT Physics Part 2: X-ray Interactions NPRE 435, Principles of Imaging with Ionizing Radiation, Fall 2006 Photoelectric Effect • Photoe- absorption is the preferred interaction for X-ray imging. 2 - •Rem.:Eb Z ; characteristic x-rays and/or Auger e preferredinhigh Zmaterial. • Probability of photoe- absorption Z3/E3 (Z = atomic no.) provide contrast according to different Z. • Due to the absorption of the incident x-ray without scatter, maximum subject contrast arises with a photoe- effect interaction No scattering contamination better contrast • Explains why contrast as higher energy x-rays are used in the imaging process - • Increased probability of photoe absorption just above the Eb of the inner shells cause discontinuities in the attenuation profiles (e.g., K-edge) NPRE 435, Principles of Imaging with Ionizing Radiation, Fall 2017 Photoelectric Effect NPRE 435, Principles of Imaging with Ionizing Radiation, Fall 2017 X-ray Cross Section and Linear Attenuation Coefficient • Cross section is a measure of the probability (‘apparent area’) of interaction: (E) measured in barns (10-24 cm2) • Interaction probability can also be expressed in terms of the thickness of the material – linear attenuation coefficient: (E) = fractional number of photons removed (attenuated) from the beam after traveling through a unit length in media by absorption or scattering -1 - • (E) [cm ]=Z[e /atom] · Navg [atoms/mole] · 1/A [moles/gm] · [gm/cm3] · (E) [cm2/e-] • Multiply by 100% to get % removed from the beam/cm • (E) as E , e.g., for soft tissue (30 keV) = 0.35 cm-1 and (100 keV) = 0.16 cm-1 NPRE 435, Principles of Imaging with Ionizing Radiation, Fall 2017 Calculation of the Linear Attenuation Coefficient To the extent that Compton scattered photons are completely removed from the beam, the attenuation coefficient can be approximated as The (effective) Z value of a material is particular important for determining . -

Exploring Density
Exploring Density Students investigate the densities of different liquids and solids and understand how density may help identify a substance. Suggested Grade Range: 6-8 Approximate Time: 1 hour Relevant National Content Standards: Next Generation Science Standards Science and Engineering Practices: Developing and using Models Modeling in 6–8 builds on K–5 experiences and progresses to developing, using, and revising models to describe, test, and predict more abstract phenomena and design systems. • Develop and use a model to describe phenomena. Science and Engineering Practices: Analyzing and Interpreting Data Analyzing data in 6-8 builds on K-5 and progresses to extending quantitative analysis to investigations, distinguishing between correlation and causation, and basic statistical techniques of data and error analysis. • Analyze and interpret data to determine similarities and differences in findings. Disciplinary Core Ideas: PS1.A Structure and Properties of Matter Each pure substance has characteristic physical and chemical properties (for any bulk quantity under given conditions) that can be used to identify it. Common Core State Standard: 7NS 2. Apply and extend previous understanding of multiplication and division and of fractions to multiply and divide rational numbers. 3. Solve real-world and mathematical problems involving the four operations with rational numbers. Common Core State Standard: 7EE Solve real-life and mathematical problems using numerical and algebraic expressions and equations. 4. Use variables to represent -

Sounds of a Supersolid A
NEWS & VIEWS RESEARCH hypothesis came from extensive population humans, implying possible mosquito exposure long-distance spread of insecticide-resistant time-series analysis from that earlier study5, to malaria parasites and the potential to spread mosquitoes, worsening an already dire situ- which showed beyond reasonable doubt that infection over great distances. ation, given the current spread of insecticide a mosquito vector species called Anopheles However, the authors failed to detect resistance in mosquito populations. This would coluzzii persists locally in the dry season in parasite infections in their aerially sampled be a matter of great concern because insecticides as-yet-undiscovered places. However, the malaria vectors, a result that they assert is to be are the best means of malaria control currently data were not consistent with this outcome for expected given the small sample size and the low available8. However, long-distance migration other malaria vectors in the study area — the parasite-infection rates typical of populations of could facilitate the desirable spread of mosqui- species Anopheles gambiae and Anopheles ara- malaria vectors. A problem with this argument toes for gene-based methods of malaria-vector biensis — leaving wind-powered long-distance is that the typical infection rates they mention control. One thing is certain, Huestis and col- migration as the only remaining possibility to are based on one specific mosquito body part leagues have permanently transformed our explain the data5. (salivary glands), rather than the unknown but understanding of African malaria vectors and Both modelling6 and genetic studies7 undoubtedly much higher infection rates that what it will take to conquer malaria. -

Package 'Constants'
Package ‘constants’ February 25, 2021 Type Package Title Reference on Constants, Units and Uncertainty Version 1.0.1 Description CODATA internationally recommended values of the fundamental physical constants, provided as symbols for direct use within the R language. Optionally, the values with uncertainties and/or units are also provided if the 'errors', 'units' and/or 'quantities' packages are installed. The Committee on Data for Science and Technology (CODATA) is an interdisciplinary committee of the International Council for Science which periodically provides the internationally accepted set of values of the fundamental physical constants. This package contains the ``2018 CODATA'' version, published on May 2019: Eite Tiesinga, Peter J. Mohr, David B. Newell, and Barry N. Taylor (2020) <https://physics.nist.gov/cuu/Constants/>. License MIT + file LICENSE Encoding UTF-8 LazyData true URL https://github.com/r-quantities/constants BugReports https://github.com/r-quantities/constants/issues Depends R (>= 3.5.0) Suggests errors (>= 0.3.6), units, quantities, testthat ByteCompile yes RoxygenNote 7.1.1 NeedsCompilation no Author Iñaki Ucar [aut, cph, cre] (<https://orcid.org/0000-0001-6403-5550>) Maintainer Iñaki Ucar <[email protected]> Repository CRAN Date/Publication 2021-02-25 13:20:05 UTC 1 2 codata R topics documented: constants-package . .2 codata . .2 lookup . .3 syms.............................................4 Index 6 constants-package constants: Reference on Constants, Units and Uncertainty Description This package provides the 2018 version of the CODATA internationally recommended values of the fundamental physical constants for their use within the R language. Author(s) Iñaki Ucar References Eite Tiesinga, Peter J. Mohr, David B.