Thermodynamics Notes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Introduction to Hydrostatics
Introduction to Hydrostatics Hydrostatics Equation The simplified Navier Stokes equation for hydrostatics is a vector equation, which can be split into three components. The convention will be adopted that gravity always acts in the negative z direction. Thus, and the three components of the hydrostatics equation reduce to Since pressure is now only a function of z, total derivatives can be used for the z-component instead of partial derivatives. In fact, this equation can be integrated directly from some point 1 to some point 2. Assuming both density and gravity remain nearly constant from 1 to 2 (a reasonable approximation unless there is a huge elevation difference between points 1 and 2), the z- component becomes Another form of this equation, which is much easier to remember is This is the only hydrostatics equation needed. It is easily remembered by thinking about scuba diving. As a diver goes down, the pressure on his ears increases. So, the pressure "below" is greater than the pressure "above." Some "rules" to remember about hydrostatics Recall, for hydrostatics, pressure can be found from the simple equation, There are several "rules" or comments which directly result from the above equation: If you can draw a continuous line through the same fluid from point 1 to point 2, then p1 = p2 if z1 = z2. For example, consider the oddly shaped container below: By this rule, p1 = p2 and p4 = p5 since these points are at the same elevation in the same fluid. However, p2 does not equal p3 even though they are at the same elevation, because one cannot draw a line connecting these points through the same fluid. -
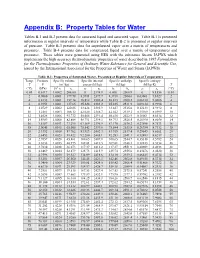
Appendix B: Property Tables for Water
Appendix B: Property Tables for Water Tables B-1 and B-2 present data for saturated liquid and saturated vapor. Table B-1 is presented information at regular intervals of temperature while Table B-2 is presented at regular intervals of pressure. Table B-3 presents data for superheated vapor over a matrix of temperatures and pressures. Table B-4 presents data for compressed liquid over a matrix of temperatures and pressures. These tables were generated using EES with the substance Steam_IAPWS which implements the high accuracy thermodynamic properties of water described in 1995 Formulation for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use, issued by the International Associated for the Properties of Water and Steam (IAPWS). Table B-1: Properties of Saturated Water, Presented at Regular Intervals of Temperature Temp. Pressure Specific volume Specific internal Specific enthalpy Specific entropy T P (m3/kg) energy (kJ/kg) (kJ/kg) (kJ/kg-K) T 3 (°C) (kPa) 10 vf vg uf ug hf hg sf sg (°C) 0.01 0.6117 1.0002 206.00 0 2374.9 0.000 2500.9 0 9.1556 0.01 2 0.7060 1.0001 179.78 8.3911 2377.7 8.3918 2504.6 0.03061 9.1027 2 4 0.8135 1.0001 157.14 16.812 2380.4 16.813 2508.2 0.06110 9.0506 4 6 0.9353 1.0001 137.65 25.224 2383.2 25.225 2511.9 0.09134 8.9994 6 8 1.0729 1.0002 120.85 33.626 2385.9 33.627 2515.6 0.12133 8.9492 8 10 1.2281 1.0003 106.32 42.020 2388.7 42.022 2519.2 0.15109 8.8999 10 12 1.4028 1.0006 93.732 50.408 2391.4 50.410 2522.9 0.18061 8.8514 12 14 1.5989 1.0008 82.804 58.791 2394.1 58.793 2526.5 -

Equation of State of an Ideal Gas
ASME231 Atmospheric Thermodynamics NC A&T State U Department of Physics Dr. Yuh-Lang Lin http://mesolab.org [email protected] Lecture 3: Equation of State of an Ideal Gas As mentioned earlier, the state of a system is the condition of the system (or part of the system) at an instant of time measured by its properties. Those properties are called state variables, such as T, p, and V. The equation which relates T, p, and V is called equation of state. Since they are related by the equation of state, only two of them are independent. Thus, all the other thermodynamic properties will depend on the state defined by two independent variables by state functions. PVT system: The simplest thermodynamic system consists of a fixed mass of a fluid uninfluenced by chemical reactions or external fields. Such a system can be described by the pressure, volume and temperature, which are related by an equation of state f (p, V, T) = 0, (2.1) 1 where only two of them are independent. From physical experiments, the equation of state for an ideal gas, which is defined as a hypothetical gas whose molecules occupy negligible space and have no interactions, may be written as p = RT, (2.2) or p = RT, (2.3) where -3 : density (=m/V) in kg m , 3 -1 : specific volume (=V/m=1/) in m kg . R: specific gas constant (R=R*/M), -1 -1 -1 -1 [R=287 J kg K for dry air (Rd), 461 J kg K for water vapor for water vapor (Rv)]. -

Thermodynamic State Variables Gunt
Fundamentals of thermodynamics 1 Thermodynamic state variables gunt Basic knowledge Thermodynamic state variables Thermodynamic systems and principles Change of state of gases In physics, an idealised model of a real gas was introduced to Equation of state for ideal gases: State variables are the measurable properties of a system. To make it easier to explain the behaviour of gases. This model is a p × V = m × Rs × T describe the state of a system at least two independent state system boundaries highly simplifi ed representation of the real states and is known · m: mass variables must be given. surroundings as an “ideal gas”. Many thermodynamic processes in gases in · Rs: spec. gas constant of the corresponding gas particular can be explained and described mathematically with State variables are e.g.: the help of this model. system • pressure (p) state process • temperature (T) • volume (V) Changes of state of an ideal gas • amount of substance (n) Change of state isochoric isobaric isothermal isentropic Condition V = constant p = constant T = constant S = constant The state functions can be derived from the state variables: Result dV = 0 dp = 0 dT = 0 dS = 0 • internal energy (U): the thermal energy of a static, closed Law p/T = constant V/T = constant p×V = constant p×Vκ = constant system. When external energy is added, processes result κ =isentropic in a change of the internal energy. exponent ∆U = Q+W · Q: thermal energy added to the system · W: mechanical work done on the system that results in an addition of heat An increase in the internal energy of the system using a pressure cooker as an example. -

Thermodynamics
ME346A Introduction to Statistical Mechanics { Wei Cai { Stanford University { Win 2011 Handout 6. Thermodynamics January 26, 2011 Contents 1 Laws of thermodynamics 2 1.1 The zeroth law . .3 1.2 The first law . .4 1.3 The second law . .5 1.3.1 Efficiency of Carnot engine . .5 1.3.2 Alternative statements of the second law . .7 1.4 The third law . .8 2 Mathematics of thermodynamics 9 2.1 Equation of state . .9 2.2 Gibbs-Duhem relation . 11 2.2.1 Homogeneous function . 11 2.2.2 Virial theorem / Euler theorem . 12 2.3 Maxwell relations . 13 2.4 Legendre transform . 15 2.5 Thermodynamic potentials . 16 3 Worked examples 21 3.1 Thermodynamic potentials and Maxwell's relation . 21 3.2 Properties of ideal gas . 24 3.3 Gas expansion . 28 4 Irreversible processes 32 4.1 Entropy and irreversibility . 32 4.2 Variational statement of second law . 32 1 In the 1st lecture, we will discuss the concepts of thermodynamics, namely its 4 laws. The most important concepts are the second law and the notion of Entropy. (reading assignment: Reif x 3.10, 3.11) In the 2nd lecture, We will discuss the mathematics of thermodynamics, i.e. the machinery to make quantitative predictions. We will deal with partial derivatives and Legendre transforms. (reading assignment: Reif x 4.1-4.7, 5.1-5.12) 1 Laws of thermodynamics Thermodynamics is a branch of science connected with the nature of heat and its conver- sion to mechanical, electrical and chemical energy. (The Webster pocket dictionary defines, Thermodynamics: physics of heat.) Historically, it grew out of efforts to construct more efficient heat engines | devices for ex- tracting useful work from expanding hot gases (http://www.answers.com/thermodynamics). -
![[5] Magnetic Densimetry: Partial Specific Volume and Other Applications](https://docslib.b-cdn.net/cover/6742/5-magnetic-densimetry-partial-specific-volume-and-other-applications-566742.webp)
[5] Magnetic Densimetry: Partial Specific Volume and Other Applications
74 MOLECULAR WEIGHT DETERMINATIONS [5] [5] Magnetic Densimetry: Partial Specific Volume and Other Applications By D. W. KUPKE and J. W. BEAMS This chapter consists of three parts. Part I is a summary of the principles and current development of the magnetic densimeter, with which density values may be obtained conveniently on small volumes of solution with the speed and accuracy required for present-day ap- plications in protein chemistry. In Part II, the applications, including definitions, are outlined whereby the density property may be utilized for the study of protein solutions. Finally, in Part III, some practical aspects on the routine determination of densities of protein solutions by magnetic densimetry are listed. Part I. Magnetic Densimeter The magnetic densimeter measures by electromagnetic methods the vertical (up or down) force on a totally immersed buoy. From these measured forces together with the known mass and volume of the buoy, the density of the solution can be obtained by employing Archimedes' principle. In practice, it is convenient to eliminate evaluation of the mass and volume of the buoy and to determine the relation of the mag- netic to the mechanical forces on the buoy by direct calibration of the latter when it is immersed in liquids of known density. Several workers have devised magnetic float methods for determin- ing densities, but the method first described by Lamb and Lee I and later improved by MacInnes, Dayhoff, and Ray: is perhaps the most accurate means (~1 part in 106) devised up to that time for determining the densities of solutions. The magnetic method was not widely used, first because it was tedious and required considerable manipulative skill; second, the buoy or float was never stationary for periods long enough to allow ruling out of wall effects, viscosity perturbations, etc.; third, the technique required comparatively large volumes of the solution (350 ml) for accurate measurements. -

Basic Thermodynamics-17ME33.Pdf
Module -1 Fundamental Concepts & Definitions & Work and Heat MODULE 1 Fundamental Concepts & Definitions Thermodynamics definition and scope, Microscopic and Macroscopic approaches. Some practical applications of engineering thermodynamic Systems, Characteristics of system boundary and control surface, examples. Thermodynamic properties; definition and units, intensive and extensive properties. Thermodynamic state, state point, state diagram, path and process, quasi-static process, cyclic and non-cyclic processes. Thermodynamic equilibrium; definition, mechanical equilibrium; diathermic wall, thermal equilibrium, chemical equilibrium, Zeroth law of thermodynamics, Temperature; concepts, scales, fixed points and measurements. Work and Heat Mechanics, definition of work and its limitations. Thermodynamic definition of work; examples, sign convention. Displacement work; as a part of a system boundary, as a whole of a system boundary, expressions for displacement work in various processes through p-v diagrams. Shaft work; Electrical work. Other types of work. Heat; definition, units and sign convention. 10 Hours 1st Hour Brain storming session on subject topics Thermodynamics definition and scope, Microscopic and Macroscopic approaches. Some practical applications of engineering thermodynamic Systems 2nd Hour Characteristics of system boundary and control surface, examples. Thermodynamic properties; definition and units, intensive and extensive properties. 3rd Hour Thermodynamic state, state point, state diagram, path and process, quasi-static -

Chapter03 Introduction to Thermodynamic Systems
Chapter03 Introduction to Thermodynamic Systems February 13, 2019 This chapter introduces the concept of thermo- Remark 1.1. In general, we will consider three kinds dynamic systems and digresses on the issue: How of exchange between S and Se through a (possibly ab- complex systems are related to Statistical Thermo- stract) membrane: dynamics. To do so, let us review the concepts of (1) Diathermal (Contrary: Adiabatic) membrane Equilibrium Thermodynamics from the conventional that allows exchange of thermal energy. viewpoint. Let U be the universe. Let a collection of objects (2) Deformable (Contrary: Rigid) membrane that S ⊆ U be defined as the system under certain restric- allows exchange of work done by volume forces tive conditions. The complement of S in U is called or surface forces. the exterior of S and is denoted as Se. (3) Permeable (Contrary: Impermeable) membrane that allows exchange of matter (e.g., in the form 1 Basic Definitions of molecules of chemical species). Definition 1.1. A system S is an idealized body that can be isolated from Se in the sense that changes in Se Definition 1.4. A thermodynamic state variable is a do not have any effects on S. macroscopic entity that is a characteristic of a system S. A thermodynamic state variable can be a scalar, Definition 1.2. A system S is called a thermody- a finite-dimensional vector, or a tensor. Examples: namic system if any possible exchange between S and temperature, or a strain tensor. Se is restricted to one or more of the following: (1) Any form of energy (e.g., thermal, electric, and magnetic) Definition 1.5. -

Chapter 3 3.4-2 the Compressibility Factor Equation of State
Chapter 3 3.4-2 The Compressibility Factor Equation of State The dimensionless compressibility factor, Z, for a gaseous species is defined as the ratio pv Z = (3.4-1) RT If the gas behaves ideally Z = 1. The extent to which Z differs from 1 is a measure of the extent to which the gas is behaving nonideally. The compressibility can be determined from experimental data where Z is plotted versus a dimensionless reduced pressure pR and reduced temperature TR, defined as pR = p/pc and TR = T/Tc In these expressions, pc and Tc denote the critical pressure and temperature, respectively. A generalized compressibility chart of the form Z = f(pR, TR) is shown in Figure 3.4-1 for 10 different gases. The solid lines represent the best curves fitted to the data. Figure 3.4-1 Generalized compressibility chart for various gases10. It can be seen from Figure 3.4-1 that the value of Z tends to unity for all temperatures as pressure approach zero and Z also approaches unity for all pressure at very high temperature. If the p, v, and T data are available in table format or computer software then you should not use the generalized compressibility chart to evaluate p, v, and T since using Z is just another approximation to the real data. 10 Moran, M. J. and Shapiro H. N., Fundamentals of Engineering Thermodynamics, Wiley, 2008, pg. 112 3-19 Example 3.4-2 ---------------------------------------------------------------------------------- A closed, rigid tank filled with water vapor, initially at 20 MPa, 520oC, is cooled until its temperature reaches 400oC. -

Thermal Equilibrium State of the World Oceans
Thermal equilibrium state of the ocean Rui Xin Huang Department of Physical Oceanography Woods Hole Oceanographic Institution Woods Hole, MA 02543, USA April 24, 2010 Abstract The ocean is in a non-equilibrium state under the external forcing, such as the mechanical energy from wind stress and tidal dissipation, plus the huge amount of thermal energy from the air-sea interface and the freshwater flux associated with evaporation and precipitation. In the study of energetics of the oceanic circulation it is desirable to examine how much energy in the ocean is available. In order to calculate the so-called available energy, a reference state should be defined. One of such reference state is the thermal equilibrium state defined in this study. 1. Introduction Chemical potential is a part of the internal energy. Thermodynamics of a multiple component system can be established from the definition of specific entropy η . Two other crucial variables of a system, including temperature and specific chemical potential, can be defined as follows 1 ⎛⎞∂η ⎛⎞∂η = ⎜⎟, μi =−Tin⎜⎟, = 1,2,..., , (1) Te m ⎝⎠∂ vm, i ⎝⎠∂ i ev, where e is the specific internal energy, v is the specific volume, mi and μi are the mass fraction and chemical potential for the i-th component. For a multiple component system, the change in total chemical potential is the sum of each component, dc , where c is the mass fraction of each component. The ∑i μii i mass fractions satisfy the constraint c 1 . Thus, the mass fraction of water in sea ∑i i = water satisfies dc=− c , and the total chemical potential for sea water is wi∑iw≠ N −1 ∑()μμiwi− dc . -

Module 2: Hydrostatics
Module 2: Hydrostatics . Hydrostatic pressure and devices: 2 lectures . Forces on surfaces: 2.5 lectures . Buoyancy, Archimedes, stability: 1.5 lectures Mech 280: Frigaard Lectures 1-2: Hydrostatic pressure . Should be able to: . Use common pressure terminology . Derive the general form for the pressure distribution in static fluid . Calculate the pressure within a constant density fluids . Calculate forces in a hydraulic press . Analyze manometers and barometers . Calculate pressure distribution in varying density fluid . Calculate pressure in fluids in rigid body motion in non-inertial frames of reference Mech 280: Frigaard Pressure . Pressure is defined as a normal force exerted by a fluid per unit area . SI Unit of pressure is N/m2, called a pascal (Pa). Since the unit Pa is too small for many pressures encountered in engineering practice, kilopascal (1 kPa = 103 Pa) and mega-pascal (1 MPa = 106 Pa) are commonly used . Other units include bar, atm, kgf/cm2, lbf/in2=psi . 1 psi = 6.695 x 103 Pa . 1 atm = 101.325 kPa = 14.696 psi . 1 bar = 100 kPa (close to atmospheric pressure) Mech 280: Frigaard Absolute, gage, and vacuum pressures . Actual pressure at a give point is called the absolute pressure . Most pressure-measuring devices are calibrated to read zero in the atmosphere. Pressure above atmospheric is called gage pressure: Pgage=Pabs - Patm . Pressure below atmospheric pressure is called vacuum pressure: Pvac=Patm - Pabs. Mech 280: Frigaard Pressure at a Point . Pressure at any point in a fluid is the same in all directions . Pressure has a magnitude, but not a specific direction, and thus it is a scalar quantity . -

Statistical Thermodynamics - Fall 2009
1 Statistical Thermodynamics - Fall 2009 Professor Dmitry Garanin Thermodynamics September 9, 2012 I. PREFACE The course of Statistical Thermodynamics consist of two parts: Thermodynamics and Statistical Physics. These both branches of physics deal with systems of a large number of particles (atoms, molecules, etc.) at equilibrium. 3 19 One cm of an ideal gas under normal conditions contains NL =2.69 10 atoms, the so-called Loschmidt number. Although one may describe the motion of the atoms with the help of× Newton’s equations, direct solution of such a large number of differential equations is impossible. On the other hand, one does not need the too detailed information about the motion of the individual particles, the microscopic behavior of the system. One is rather interested in the macroscopic quantities, such as the pressure P . Pressure in gases is due to the bombardment of the walls of the container by the flying atoms of the contained gas. It does not exist if there are only a few gas molecules. Macroscopic quantities such as pressure arise only in systems of a large number of particles. Both thermodynamics and statistical physics study macroscopic quantities and relations between them. Some macroscopics quantities, such as temperature and entropy, are non-mechanical. Equilibruim, or thermodynamic equilibrium, is the state of the system that is achieved after some time after time-dependent forces acting on the system have been switched off. One can say that the system approaches the equilibrium, if undisturbed. Again, thermodynamic equilibrium arises solely in macroscopic systems. There is no thermodynamic equilibrium in a system of a few particles that are moving according to the Newton’s law.