Ideal Gasses Is Known As the Ideal Gas Law
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Simple Experiment Confirming the Negative Temperature Dependence of Gravity Force
SIMPLE EXPERIMENT CONFIRMING THE NEGATIVE TEMPERATURE DEPENDENCE OF GRAVITY FORCE Alexander L. Dmitriev St-Petersburg National Research University of Information Technologies, Mechanics and Optics, 49 Kronverksky Prospect, St-Petersburg, 197101, Russia Results of weighing of the tight vessel containing a thermo-isolated copper sample heated by a tungstic spiral are submitted. The increase of temperature of a sample with masse of 28 g for about 10 0 С causes a reduction of its apparent weight for 0.7 mg. The basic sources of measurement errors are briefly considered, the expediency of researches of temperature dependence of gravity is recognized. Key words: gravity force, weight, temperature, gravity mass. PACS: 04.80-y. Introduction The question on influence of temperature of bodies on the force of their gravitational interaction was already raised from the times when the law of gravitation was formulated. The first exact experiments in this area were carried out at the end of XIXth - the beginning of XXth century with the purpose of checking the consequences of various electromagnetic theories of gravitation (Mi, Weber, Morozov) according to which the force of a gravitational attraction of bodies is increased with the growth of their absolute temperature [1-3]. That period of experimental researches was completed in 1923 by the publication of Shaw and Davy’s work who concluded that the temperature dependence of gravitation forces does not exceed relative value of 2⋅10 −6 K −1 and can be is equal to zero [4]. Actually, as shown in [5], those authors confidently registered the negative temperature dependence of gravitation force. -

Solutes and Solution
Solutes and Solution The first rule of solubility is “likes dissolve likes” Polar or ionic substances are soluble in polar solvents Non-polar substances are soluble in non- polar solvents Solutes and Solution There must be a reason why a substance is soluble in a solvent: either the solution process lowers the overall enthalpy of the system (Hrxn < 0) Or the solution process increases the overall entropy of the system (Srxn > 0) Entropy is a measure of the amount of disorder in a system—entropy must increase for any spontaneous change 1 Solutes and Solution The forces that drive the dissolution of a solute usually involve both enthalpy and entropy terms Hsoln < 0 for most species The creation of a solution takes a more ordered system (solid phase or pure liquid phase) and makes more disordered system (solute molecules are more randomly distributed throughout the solution) Saturation and Equilibrium If we have enough solute available, a solution can become saturated—the point when no more solute may be accepted into the solvent Saturation indicates an equilibrium between the pure solute and solvent and the solution solute + solvent solution KC 2 Saturation and Equilibrium solute + solvent solution KC The magnitude of KC indicates how soluble a solute is in that particular solvent If KC is large, the solute is very soluble If KC is small, the solute is only slightly soluble Saturation and Equilibrium Examples: + - NaCl(s) + H2O(l) Na (aq) + Cl (aq) KC = 37.3 A saturated solution of NaCl has a [Na+] = 6.11 M and [Cl-] = -

Thermodynamics Notes
Thermodynamics Notes Steven K. Krueger Department of Atmospheric Sciences, University of Utah August 2020 Contents 1 Introduction 1 1.1 What is thermodynamics? . .1 1.2 The atmosphere . .1 2 The Equation of State 1 2.1 State variables . .1 2.2 Charles' Law and absolute temperature . .2 2.3 Boyle's Law . .3 2.4 Equation of state of an ideal gas . .3 2.5 Mixtures of gases . .4 2.6 Ideal gas law: molecular viewpoint . .6 3 Conservation of Energy 8 3.1 Conservation of energy in mechanics . .8 3.2 Conservation of energy: A system of point masses . .8 3.3 Kinetic energy exchange in molecular collisions . .9 3.4 Working and Heating . .9 4 The Principles of Thermodynamics 11 4.1 Conservation of energy and the first law of thermodynamics . 11 4.1.1 Conservation of energy . 11 4.1.2 The first law of thermodynamics . 11 4.1.3 Work . 12 4.1.4 Energy transferred by heating . 13 4.2 Quantity of energy transferred by heating . 14 4.3 The first law of thermodynamics for an ideal gas . 15 4.4 Applications of the first law . 16 4.4.1 Isothermal process . 16 4.4.2 Isobaric process . 17 4.4.3 Isosteric process . 18 4.5 Adiabatic processes . 18 5 The Thermodynamics of Water Vapor and Moist Air 21 5.1 Thermal properties of water substance . 21 5.2 Equation of state of moist air . 21 5.3 Mixing ratio . 22 5.4 Moisture variables . 22 5.5 Changes of phase and latent heats . -

Entropy: Ideal Gas Processes
Chapter 19: The Kinec Theory of Gases Thermodynamics = macroscopic picture Gases micro -> macro picture One mole is the number of atoms in 12 g sample Avogadro’s Number of carbon-12 23 -1 C(12)—6 protrons, 6 neutrons and 6 electrons NA=6.02 x 10 mol 12 atomic units of mass assuming mP=mn Another way to do this is to know the mass of one molecule: then So the number of moles n is given by M n=N/N sample A N = N A mmole−mass € Ideal Gas Law Ideal Gases, Ideal Gas Law It was found experimentally that if 1 mole of any gas is placed in containers that have the same volume V and are kept at the same temperature T, approximately all have the same pressure p. The small differences in pressure disappear if lower gas densities are used. Further experiments showed that all low-density gases obey the equation pV = nRT. Here R = 8.31 K/mol ⋅ K and is known as the "gas constant." The equation itself is known as the "ideal gas law." The constant R can be expressed -23 as R = kNA . Here k is called the Boltzmann constant and is equal to 1.38 × 10 J/K. N If we substitute R as well as n = in the ideal gas law we get the equivalent form: NA pV = NkT. Here N is the number of molecules in the gas. The behavior of all real gases approaches that of an ideal gas at low enough densities. Low densitiens m= enumberans tha oft t hemoles gas molecul es are fa Nr e=nough number apa ofr tparticles that the y do not interact with one another, but only with the walls of the gas container. -
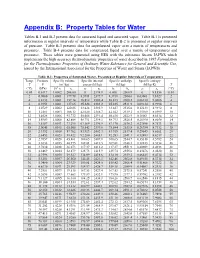
Appendix B: Property Tables for Water
Appendix B: Property Tables for Water Tables B-1 and B-2 present data for saturated liquid and saturated vapor. Table B-1 is presented information at regular intervals of temperature while Table B-2 is presented at regular intervals of pressure. Table B-3 presents data for superheated vapor over a matrix of temperatures and pressures. Table B-4 presents data for compressed liquid over a matrix of temperatures and pressures. These tables were generated using EES with the substance Steam_IAPWS which implements the high accuracy thermodynamic properties of water described in 1995 Formulation for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use, issued by the International Associated for the Properties of Water and Steam (IAPWS). Table B-1: Properties of Saturated Water, Presented at Regular Intervals of Temperature Temp. Pressure Specific volume Specific internal Specific enthalpy Specific entropy T P (m3/kg) energy (kJ/kg) (kJ/kg) (kJ/kg-K) T 3 (°C) (kPa) 10 vf vg uf ug hf hg sf sg (°C) 0.01 0.6117 1.0002 206.00 0 2374.9 0.000 2500.9 0 9.1556 0.01 2 0.7060 1.0001 179.78 8.3911 2377.7 8.3918 2504.6 0.03061 9.1027 2 4 0.8135 1.0001 157.14 16.812 2380.4 16.813 2508.2 0.06110 9.0506 4 6 0.9353 1.0001 137.65 25.224 2383.2 25.225 2511.9 0.09134 8.9994 6 8 1.0729 1.0002 120.85 33.626 2385.9 33.627 2515.6 0.12133 8.9492 8 10 1.2281 1.0003 106.32 42.020 2388.7 42.022 2519.2 0.15109 8.8999 10 12 1.4028 1.0006 93.732 50.408 2391.4 50.410 2522.9 0.18061 8.8514 12 14 1.5989 1.0008 82.804 58.791 2394.1 58.793 2526.5 -

Weight and Lifestyle Inventory (Wali)
WEIGHT AND LIFESTYLE INVENTORY (Bariatric Surgery Version) © 2015 Thomas A. Wadden, Ph.D. and Gary D. Foster, Ph.D. 1 The Weight and Lifestyle Inventory (WALI) is designed to obtain information about your weight and dieting histories, your eating and exercise habits, and your relationships with family and friends. Please complete the questionnaire carefully and make your best guess when unsure of the answer. You will have an opportunity to review your answers with a member of our professional staff. Please allow 30-60 minutes to complete this questionnaire. Your answers will help us better identify problem areas and plan your treatment accordingly. The information you provide will become part of your medical record at Penn Medicine and may be shared with members of our treatment team. Thank you for taking the time to complete this questionnaire. SECTION A: IDENTIFYING INFORMATION ______________________________________________________________________________ 1 Name _________________________ __________ _______lbs. ________ft. ______inches 2 Date of Birth 3 Age 4 Weight 5 Height ______________________________________________________________________________ 6 Address ____________________ ________________________ ______________________/_______ yrs. 7 Phone: Cell 8 Phone: Home 9 Occupation/# of yrs. at job __________________________ 10 Today’s Date 11 Highest year of school completed: (Check one.) □ 6 □ 7 □ 8 □ 9 □ 10 □ 11 □ 12 □ 13 □ 14 □ 15 □ 16 □ Masters □ Doctorate Middle School High School College 12 Race (Check all that apply): □ American Indian □ Asian □ African American/Black □ Pacific Islander □White □ Other: ______________ 13 Are you Latino, Hispanic, or of Spanish origin? □ Yes □ No SECTION B: WEIGHT HISTORY 1. At what age were you first overweight by 10 lbs. or more? _______ yrs. old 2. What has been your highest weight after age 21? _______ lbs. -

Equation of State of an Ideal Gas
ASME231 Atmospheric Thermodynamics NC A&T State U Department of Physics Dr. Yuh-Lang Lin http://mesolab.org [email protected] Lecture 3: Equation of State of an Ideal Gas As mentioned earlier, the state of a system is the condition of the system (or part of the system) at an instant of time measured by its properties. Those properties are called state variables, such as T, p, and V. The equation which relates T, p, and V is called equation of state. Since they are related by the equation of state, only two of them are independent. Thus, all the other thermodynamic properties will depend on the state defined by two independent variables by state functions. PVT system: The simplest thermodynamic system consists of a fixed mass of a fluid uninfluenced by chemical reactions or external fields. Such a system can be described by the pressure, volume and temperature, which are related by an equation of state f (p, V, T) = 0, (2.1) 1 where only two of them are independent. From physical experiments, the equation of state for an ideal gas, which is defined as a hypothetical gas whose molecules occupy negligible space and have no interactions, may be written as p = RT, (2.2) or p = RT, (2.3) where -3 : density (=m/V) in kg m , 3 -1 : specific volume (=V/m=1/) in m kg . R: specific gas constant (R=R*/M), -1 -1 -1 -1 [R=287 J kg K for dry air (Rd), 461 J kg K for water vapor for water vapor (Rv)]. -

THE SOLUBILITY of GASES in LIQUIDS Introductory Information C
THE SOLUBILITY OF GASES IN LIQUIDS Introductory Information C. L. Young, R. Battino, and H. L. Clever INTRODUCTION The Solubility Data Project aims to make a comprehensive search of the literature for data on the solubility of gases, liquids and solids in liquids. Data of suitable accuracy are compiled into data sheets set out in a uniform format. The data for each system are evaluated and where data of sufficient accuracy are available values are recommended and in some cases a smoothing equation is given to represent the variation of solubility with pressure and/or temperature. A text giving an evaluation and recommended values and the compiled data sheets are published on consecutive pages. The following paper by E. Wilhelm gives a rigorous thermodynamic treatment on the solubility of gases in liquids. DEFINITION OF GAS SOLUBILITY The distinction between vapor-liquid equilibria and the solubility of gases in liquids is arbitrary. It is generally accepted that the equilibrium set up at 300K between a typical gas such as argon and a liquid such as water is gas-liquid solubility whereas the equilibrium set up between hexane and cyclohexane at 350K is an example of vapor-liquid equilibrium. However, the distinction between gas-liquid solubility and vapor-liquid equilibrium is often not so clear. The equilibria set up between methane and propane above the critical temperature of methane and below the criti cal temperature of propane may be classed as vapor-liquid equilibrium or as gas-liquid solubility depending on the particular range of pressure considered and the particular worker concerned. -

On the Equation of State of an Ideal Monatomic Gas According to the Quantum-Theory, In: KNAW, Proceedings, 16 I, 1913, Amsterdam, 1913, Pp
Huygens Institute - Royal Netherlands Academy of Arts and Sciences (KNAW) Citation: W.H. Keesom, On the equation of state of an ideal monatomic gas according to the quantum-theory, in: KNAW, Proceedings, 16 I, 1913, Amsterdam, 1913, pp. 227-236 This PDF was made on 24 September 2010, from the 'Digital Library' of the Dutch History of Science Web Center (www.dwc.knaw.nl) > 'Digital Library > Proceedings of the Royal Netherlands Academy of Arts and Sciences (KNAW), http://www.digitallibrary.nl' - 1 - 227 high. We are uncertain as to the cause of this differencc: most probably it is due to an nncertainty in the temperature with the absolute manometer. It is of special interest to compare these observations with NERNST'S formula. Tbe fourth column of Table II contains the pressUl'es aceord ing to this formula, calc111ated witb the eonstants whieb FALCK 1) ha~ determined with the data at bis disposal. FALOK found the following expression 6000 1 0,009983 log P • - --. - + 1. 7 5 log T - T + 3,1700 4,571 T . 4,571 where p is the pressure in atmospheres. The correspondence will be se en to be satisfactory considering the degree of accuracy of the observations. 1t does riot look as if the constants could be materially improved. Physics. - "On the equation of state oj an ideal monatomic gflS accoJ'ding to tlw quantum-the01'Y." By Dr. W. H. KEEsmr. Supple ment N°. 30a to the Oommunications ft'om the PhysicaL Labora tory at Leiden. Oommunicated by Prof. H. KAl\IERLINGH ONNES. (Communicated in the meeting of May' 31, 1913). -

AP Chemistry Chapter 5 Gases Ch
AP Chemistry Chapter 5 Gases Ch. 5 Gases Agenda 23 September 2017 Per 3 & 5 ○ 5.1 Pressure ○ 5.2 The Gas Laws ○ 5.3 The Ideal Gas Law ○ 5.4 Gas Stoichiometry ○ 5.5 Dalton’s Law of Partial Pressures ○ 5.6 The Kinetic Molecular Theory of Gases ○ 5.7 Effusion and Diffusion ○ 5.8 Real Gases ○ 5.9 Characteristics of Several Real Gases ○ 5.10 Chemistry in the Atmosphere 5.1 Units of Pressure 760 mm Hg = 760 Torr = 1 atm Pressure = Force = Newton = pascal (Pa) Area m2 5.2 Gas Laws of Boyle, Charles and Avogadro PV = k Slope = k V = k P y = k(1/P) + 0 5.2 Gas Laws of Boyle - use the actual data from Boyle’s Experiment Table 5.1 And Desmos to plot Volume vs. Pressure And then Pressure vs 1/V. And PV vs. V And PV vs. P Boyle’s Law 3 centuries later? Boyle’s law only holds precisely at very low pressures.Measurements at higher pressures reveal PV is not constant, but varies as pressure varies. Deviations slight at pressures close to 1 atm we assume gases obey Boyle’s law in our calcs. A gas that strictly obeys Boyle’s Law is called an Ideal gas. Extrapolate (extend the line beyond the experimental points) back to zero pressure to find “ideal” value for k, 22.41 L atm Look Extrapolatefamiliar? (extend the line beyond the experimental points) back to zero pressure to find “ideal” value for k, 22.41 L atm The volume of a gas at constant pressure increases linearly with the Charles’ Law temperature of the gas. -

THE SOLUBILITY of GASES in LIQUIDS INTRODUCTION the Solubility Data Project Aims to Make a Comprehensive Search of the Lit- Erat
THE SOLUBILITY OF GASES IN LIQUIDS R. Battino, H. L. Clever and C. L. Young INTRODUCTION The Solubility Data Project aims to make a comprehensive search of the lit erature for data on the solubility of gases, liquids and solids in liquids. Data of suitable accuracy are compiled into data sheets set out in a uni form format. The data for each system are evaluated and where data of suf ficient accuracy are available values recommended and in some cases a smoothing equation suggested to represent the variation of solubility with pressure and/or temperature. A text giving an evaluation and recommended values and the compiled data sheets are pUblished on consecutive pages. DEFINITION OF GAS SOLUBILITY The distinction between vapor-liquid equilibria and the solUbility of gases in liquids is arbitrary. It is generally accepted that the equilibrium set up at 300K between a typical gas such as argon and a liquid such as water is gas liquid solubility whereas the equilibrium set up between hexane and cyclohexane at 350K is an example of vapor-liquid equilibrium. However, the distinction between gas-liquid solUbility and vapor-liquid equilibrium is often not so clear. The equilibria set up between methane and propane above the critical temperature of methane and below the critical temperature of propane may be classed as vapor-liquid equilibrium or as gas-liquid solu bility depending on the particular range of pressure considered and the par ticular worker concerned. The difficulty partly stems from our inability to rigorously distinguish between a gas, a vapor, and a liquid, which has been discussed in numerous textbooks. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S.