Ideal Gas Law PV = Nrt R = Universal Gas Constant R = 0.08206 L-Atm R = 8.314 J Mol-K Mol-K Example
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Solutes and Solution
Solutes and Solution The first rule of solubility is “likes dissolve likes” Polar or ionic substances are soluble in polar solvents Non-polar substances are soluble in non- polar solvents Solutes and Solution There must be a reason why a substance is soluble in a solvent: either the solution process lowers the overall enthalpy of the system (Hrxn < 0) Or the solution process increases the overall entropy of the system (Srxn > 0) Entropy is a measure of the amount of disorder in a system—entropy must increase for any spontaneous change 1 Solutes and Solution The forces that drive the dissolution of a solute usually involve both enthalpy and entropy terms Hsoln < 0 for most species The creation of a solution takes a more ordered system (solid phase or pure liquid phase) and makes more disordered system (solute molecules are more randomly distributed throughout the solution) Saturation and Equilibrium If we have enough solute available, a solution can become saturated—the point when no more solute may be accepted into the solvent Saturation indicates an equilibrium between the pure solute and solvent and the solution solute + solvent solution KC 2 Saturation and Equilibrium solute + solvent solution KC The magnitude of KC indicates how soluble a solute is in that particular solvent If KC is large, the solute is very soluble If KC is small, the solute is only slightly soluble Saturation and Equilibrium Examples: + - NaCl(s) + H2O(l) Na (aq) + Cl (aq) KC = 37.3 A saturated solution of NaCl has a [Na+] = 6.11 M and [Cl-] = -

Ideal Gasses Is Known As the Ideal Gas Law
ESCI 341 – Atmospheric Thermodynamics Lesson 4 –Ideal Gases References: An Introduction to Atmospheric Thermodynamics, Tsonis Introduction to Theoretical Meteorology, Hess Physical Chemistry (4th edition), Levine Thermodynamics and an Introduction to Thermostatistics, Callen IDEAL GASES An ideal gas is a gas with the following properties: There are no intermolecular forces, except during collisions. All collisions are elastic. The individual gas molecules have no volume (they behave like point masses). The equation of state for ideal gasses is known as the ideal gas law. The ideal gas law was discovered empirically, but can also be derived theoretically. The form we are most familiar with, pV nRT . Ideal Gas Law (1) R has a value of 8.3145 J-mol1-K1, and n is the number of moles (not molecules). A true ideal gas would be monatomic, meaning each molecule is comprised of a single atom. Real gasses in the atmosphere, such as O2 and N2, are diatomic, and some gasses such as CO2 and O3 are triatomic. Real atmospheric gasses have rotational and vibrational kinetic energy, in addition to translational kinetic energy. Even though the gasses that make up the atmosphere aren’t monatomic, they still closely obey the ideal gas law at the pressures and temperatures encountered in the atmosphere, so we can still use the ideal gas law. FORM OF IDEAL GAS LAW MOST USED BY METEOROLOGISTS In meteorology we use a modified form of the ideal gas law. We first divide (1) by volume to get n p RT . V we then multiply the RHS top and bottom by the molecular weight of the gas, M, to get Mn R p T . -
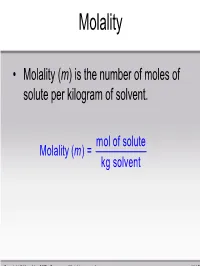
Mol of Solute Molality ( ) = Kg Solvent M
Molality • Molality (m) is the number of moles of solute per kilogram of solvent. mol of solute Molality (m ) = kg solvent Copyright © Houghton Mifflin Company. All rights reserved. 11 | 51 Sample Problem Calculate the molality of a solution of 13.5g of KF dissolved in 250. g of water. mol of solute m = kg solvent 1 m ol KF ()13.5g 58.1 = 0.250 kg = 0.929 m Copyright © Houghton Mifflin Company. All rights reserved. 11 | 52 Mole Fraction • Mole fraction (χ) is the ratio of the number of moles of a substance over the total number of moles of substances in solution. number of moles of i χ = i total number of moles ni = nT Copyright © Houghton Mifflin Company. All rights reserved. 11 | 53 Sample Problem -Conversions between units- • ex) What is the molality of a 0.200 M aluminum nitrate solution (d = 1.012g/mL)? – Work with 1 liter of solution. mass = 1012 g – mass Al(NO3)3 = 0.200 mol × 213.01 g/mol = 42.6 g ; – mass water = 1012 g -43 g = 969 g 0.200mol Molality==0.206 mol / kg 0.969kg Copyright © Houghton Mifflin Company. All rights reserved. 11 | 54 Sample Problem Calculate the mole fraction of 10.0g of NaCl dissolved in 100. g of water. mol of NaCl χ=NaCl mol of NaCl + mol H2 O 1 mol NaCl ()10.0g 58.5g NaCl = 1 m ol NaCl 1 m ol H2 O ()10.0g+ () 100.g H2 O 58.5g NaCl 18.0g H2 O = 0.0299 Copyright © Houghton Mifflin Company. -

Molar Gas Constant
Molar gas constant The molar gas constant (also known as the universal or ideal gas constant and designated by the symbol R ) is an important physical constant which appears in many of the fundamental equations inphysics, chemistry and engineering, such as the ideal gas law, other equations of state and the Nernst equation. R is equivalent to the Boltzmann constant (designated kB) times Avogadro's constant (designated NA) and thus: R = kBNA. Currently the most accurate value as published by the National Institute of Standards and Technology (NIST) is:[1] R = 8.3144621 J · K–1 · mol–1 A number of values of R in other units are provided in the adjacent table. The gas constant occurs in the ideal gas law as follows: PV = nRT or as: PVm = RT where: P is the absolute pressure of the gas T is the absolute temperature of the gas V is the volume the gas occupies n is the number of moles of gas Vm is the molar volume of the gas The specific gas constant The gas constant of a specific gas, as differentiated from the above universal molar gas constant which applies for any ideal gas, is designated by the symbol Rs and is equal to the molar gas constant divided by the molecular mass (M ) of the gas: Rs = R ÷ M The specific gas constant for an ideal gas may also be obtained from the following thermodynamicsrelationship:[2] Rs = cp – cv where cp and cv are the gas's specific heats at constant pressure and constant volume respectively. Some example values of the specific gas constant are: –1 –1 –1 Ammonia (molecular mass of 17.032 g · mol ) : Rs = 0.4882 J · K · g –1 –1 –1 Hydrogen (molecular mass of 2.016 g · mol ) : Rs = 4.1242 J · K · g –1 –1 –1 Methane (molecular mass of 16.043 g · mol ) : Rs = 0.5183 J · K · g Unfortunately, many authors in the technical literature sometimes use R as the specific gas constant without designating it as such or stating that it is the specific gas constant. -

Thermodynamic Temperature
Thermodynamic temperature Thermodynamic temperature is the absolute measure 1 Overview of temperature and is one of the principal parameters of thermodynamics. Temperature is a measure of the random submicroscopic Thermodynamic temperature is defined by the third law motions and vibrations of the particle constituents of of thermodynamics in which the theoretically lowest tem- matter. These motions comprise the internal energy of perature is the null or zero point. At this point, absolute a substance. More specifically, the thermodynamic tem- zero, the particle constituents of matter have minimal perature of any bulk quantity of matter is the measure motion and can become no colder.[1][2] In the quantum- of the average kinetic energy per classical (i.e., non- mechanical description, matter at absolute zero is in its quantum) degree of freedom of its constituent particles. ground state, which is its state of lowest energy. Thermo- “Translational motions” are almost always in the classical dynamic temperature is often also called absolute tem- regime. Translational motions are ordinary, whole-body perature, for two reasons: one, proposed by Kelvin, that movements in three-dimensional space in which particles it does not depend on the properties of a particular mate- move about and exchange energy in collisions. Figure 1 rial; two that it refers to an absolute zero according to the below shows translational motion in gases; Figure 4 be- properties of the ideal gas. low shows translational motion in solids. Thermodynamic temperature’s null point, absolute zero, is the temperature The International System of Units specifies a particular at which the particle constituents of matter are as close as scale for thermodynamic temperature. -

Chemical Engineering Thermodynamics
CHEMICAL ENGINEERING THERMODYNAMICS Andrew S. Rosen SYMBOL DICTIONARY | 1 TABLE OF CONTENTS Symbol Dictionary ........................................................................................................................ 3 1. Measured Thermodynamic Properties and Other Basic Concepts .................................. 5 1.1 Preliminary Concepts – The Language of Thermodynamics ........................................................ 5 1.2 Measured Thermodynamic Properties .......................................................................................... 5 1.2.1 Volume .................................................................................................................................................... 5 1.2.2 Temperature ............................................................................................................................................. 5 1.2.3 Pressure .................................................................................................................................................... 6 1.3 Equilibrium ................................................................................................................................... 7 1.3.1 Fundamental Definitions .......................................................................................................................... 7 1.3.2 Independent and Dependent Thermodynamic Properties ........................................................................ 7 1.3.3 Phases ..................................................................................................................................................... -

Molal Freezing Point Depression Constants SCIENTIFIC Calculating Kf Values
Molal Freezing Point Depression Constants SCIENTIFIC Calculating Kf Values Introduction The freezing point of a liquid is the temperature at which the forces of attraction among molecules are just enough to cause a phase change from the liquid state to the solid state. It is the temperature at which the liquid and solid phases exist in equilib- rium. The solvent molecules in a solution are somewhat more separated from each other than they are in a pure solvent due to the interference of solute particles. Therefore, the temperature of a solution must be lowered below the freezing point of the pure solvent in order to freeze it. The freezing point depression (∆Tf) of solutions of nonelectrolytes has been found to be equal to the molality (m) of the solute times a proportionality constant called the molal freezing point depression constant (Kf). Therefore, ∆Tf = Kf × m, Equation 1 where m is the number of moles of solute per kilograms of solvent. Concepts • Freezing point depression • Colligative properties Discussion The determination of the molecular mass of a substance from the freezing point depression is included in many high school and most college laboratory manuals. In a typical experiment, the freezing point of the pure solvent is measured followed by the freezing point of a solution of known molality. Dividing the freezing point depression (∆Tf) by the solution molality (m) gives the molal freezing point constant (Kf) of the solvent, according to Equation 1. The second part of the lab consists of using the Kf value determined in part one to determine the molality, and ultimately the molecular mass of an unknown substance. -

Ideal Gas Law
ATMO 551a Fall 2010 Ideal gas law The ideal gas law is one of the great achievements of Thermodynamics. It relates the pressure, temperature and density of gases. It is VERY close to true for typical atmospheric conditions. It assumes the molecules interact like billiard balls. For water vapor with its large permanent electric dipole moment, it does not quite behave as an ideal gas but even it is close. The microscopic version of the ideal gas law is P = n kB T where P is pressure in pascals, n is the number density of the gas in molecules per cubic meter -23 and T is the temperature in Kelvin and kB is Boltzmann’s constant = 1.38x10 J/K. For typical atmospheric conditions, n is huge and kB is always tiny. So there is a different “macroscopic” version of the ideal gas law with numbers that are easier to work with. Avogadro’s number is the number of molecules in a “mole” where a mole is defined such that 1 mole of a molecules has a mass in grams equal to the mass of an individual molecule in atomic mass units (amu). For example, 1 mole of hydrogen atoms has a mass of ~1 gram. The number 23 -1 of molecules in a mole is given by Avogadro’s number, NA = 6.02214179(30)×10 mol . We multiply and divide the first equation by NA to get P = n kB T = n/NA kBNA T = N R* T where N is the number of moles per unit volume = n/NA and R* is the ideal gas constant = kB NA. -

The Properties of Helium: Density, Specific Heats, Viscosity, and Thermal Conductivity at Pressures from 1 to 100 Bar and from Room Temperature to About 1800 K
Downloaded from orbit.dtu.dk on: Oct 04, 2021 The properties of helium: Density, specific heats, viscosity, and thermal conductivity at pressures from 1 to 100 bar and from room temperature to about 1800 K Petersen, H. Publication date: 1970 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Petersen, H. (1970). The properties of helium: Density, specific heats, viscosity, and thermal conductivity at pressures from 1 to 100 bar and from room temperature to about 1800 K. Risø National Laboratory. Denmark. Forskningscenter Risoe. Risoe-R No. 224 General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Users may download and print one copy of any publication from the public portal for the purpose of private study or research. You may not further distribute the material or use it for any profit-making activity or commercial gain You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. « Riso Report No. 224 5 o, « Danish Atomic Energy Commission .1 * Research Establishment Ris6 The Properties of Helium: Density, Specific Heats, Viscosity, and Thermal Conductivity at Pressures from 1 to 100 bar and from Room Temperature to about 1800 K by Helge Petersen September, 1970 Sain dUiributort: JUL GjiUtrap, 17, »Wpdt, DK-U07 Capubaftn K, Dnaurk U.D.C m»!:62U34.3t September, 1 970 Rise Report No. -
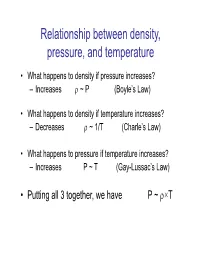
Relationship Between Density, Pressure, and Temperature
Relationship between density, pressure, and temperature • What happens to density if pressure increases? – Increases ~ P (Boyle’s Law) • What happens to density if temperature increases? – Decreases ~ 1/T (Charle’s Law) • What happens to pressure if temperature increases? – Increases P ~ T (Gay-Lussac’s Law) • Putting all 3 together, we have P ~ ×T Relationship between density, pressure, and temperature • The ideal gas law for dry air P RdT Stull (1.12) – Rd: gas constant for dry air • Equals to 287 J/kg/K – Note that P, , and T have to be in S.I. units for this equation to work using this value of Rd Numerical example • What is the pressure of dry air with a temperature of 10 oC and a density of 1kg/m3? – Use the ideal gas law: P = Rd T – Need to express all quantities in S.I. units • T = 10 oC = 10+273.15 K = 283.15 K • = 1 kg/m3 already in S.I. units –p = RdT = 1x287x283.15 = 81264 Pa Classwork example • What is the density of dry air with a temperature of 20 oC and a pressure of 800 hPa? – Use the ideal gas law: P = Rd T – Need to express all quantities in S.I. units • T = 20 oC = 20+273.15 K = 293.15 K • P = 800 hPa = 800 x 100 Pa = 80000 Pa 3 – = P/Rd/T = 80000/287/293.15 = 0.95 kg/m • For moist air: – Question for thought: At the same temperature and pressure, is moist air more dense or less dense than dry air? –Answer: Less dense! • At the same temperature and pressure, the same volume of gas contains the same number of molecules. -

6—Evaluation of the Gas Law Constant
6—Evaluation of the Gas Law Constant Name: ______________________________________________ Date: ________________________________________________ Section: _____________________________________________ Objectives • Measure the value of the gas constant R • Use Dalton’s Law to calculate the partial pressure of hydrogen in a closed container • Learn to collect a gas by displacing water from a closed container • Additional experience with the uncertainty of physical measurements • Additional experience with significant figures • Additional experience with average, deviation, average deviation and relative average deviation Pre-Laboratory Requirements • Read chapter 5.1-5.4 in Silberberg • Watch the instructional video titled “Eudiometer” • Pre-lab questions (if required by your instructor) • Laboratory notebook—prepared before lab (if required by your instructor) Safety Notes • Eye protection must be worn at all times. • Hydrochloric acid is caustic and should not come in contact with your skin or clothing. Wear gloves when handling hydrochloric acid. A lab coat or lab apron is recommended. • Hydrogen is highly flammable; there should be no open flames or electrical sparks in the area Discussion Gases, liquids, and solids constitute the three common forms of matter. This experiment gives you the opportunity to explore several properties of gases and to measure a value for the international gas constant R. Gases expand to occupy the container in which they are placed and any gas is less dense than the liquid or solid form of a substance. In addition to expanding to completely fill containers, gases exert pressure on the walls of the container and the pressure increases with increasing temperature. The relationships between volume, pressure, and temperature are summarized in Charles’ law in the Boyle’s law. -

P = PRT Or Pv = RT
Department of Earth & Climate Sciences Name_____________________ San Francisco State University In-Class Exercise/Lab 4 and Homework 4: Understanding the Gas Law 100 points (Due Monday 23 February 2015) The “simplified” or conceptual (explained in class) Ideal Gas Law (Equation of State) is: p = ρRT or (1a, b) pV = RT where p is pressure, ρ is density, R is a constant called the “gas constant” T is temperature. In the alternate version at the bottom, V is specific volume, which is the volume a unit amount of Gas, in this case 1 kiloGram, occupies. You can just think of this as “volume”. In reality, specific volume and density are inversely related. 1 V = ρ (2) As you can reason out, in the MKS system, the units of specific volume are m3 kg-1 and of density, kg m-3. Answer in complete sentences and on separate sheets. Part A. Concept ForminG 1. Examine equation 1(a). In a situation in which temperature remains constant, are pressure and density directly or inversely related? Explain? (Answer in complete sentences) (10 points) In a situation in which temperature remains constant, pressure is directly related to density. Since R and T are constant, to keep the equality, as density goes up, pressure must go up. 2. Examine equation 1(b). In situation in which volume remains constant, are pressure and temperature directly or inversely related? Explain. (10 points) In a situation in which volume (density) remains constant, pressure and temperature are directly related. Since R and V (or rho) are constant, as temperature goes up, pressure must go up.