The Ideal Gas Constant Author: John M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Simple Experiment Confirming the Negative Temperature Dependence of Gravity Force
SIMPLE EXPERIMENT CONFIRMING THE NEGATIVE TEMPERATURE DEPENDENCE OF GRAVITY FORCE Alexander L. Dmitriev St-Petersburg National Research University of Information Technologies, Mechanics and Optics, 49 Kronverksky Prospect, St-Petersburg, 197101, Russia Results of weighing of the tight vessel containing a thermo-isolated copper sample heated by a tungstic spiral are submitted. The increase of temperature of a sample with masse of 28 g for about 10 0 С causes a reduction of its apparent weight for 0.7 mg. The basic sources of measurement errors are briefly considered, the expediency of researches of temperature dependence of gravity is recognized. Key words: gravity force, weight, temperature, gravity mass. PACS: 04.80-y. Introduction The question on influence of temperature of bodies on the force of their gravitational interaction was already raised from the times when the law of gravitation was formulated. The first exact experiments in this area were carried out at the end of XIXth - the beginning of XXth century with the purpose of checking the consequences of various electromagnetic theories of gravitation (Mi, Weber, Morozov) according to which the force of a gravitational attraction of bodies is increased with the growth of their absolute temperature [1-3]. That period of experimental researches was completed in 1923 by the publication of Shaw and Davy’s work who concluded that the temperature dependence of gravitation forces does not exceed relative value of 2⋅10 −6 K −1 and can be is equal to zero [4]. Actually, as shown in [5], those authors confidently registered the negative temperature dependence of gravitation force. -

Solutes and Solution
Solutes and Solution The first rule of solubility is “likes dissolve likes” Polar or ionic substances are soluble in polar solvents Non-polar substances are soluble in non- polar solvents Solutes and Solution There must be a reason why a substance is soluble in a solvent: either the solution process lowers the overall enthalpy of the system (Hrxn < 0) Or the solution process increases the overall entropy of the system (Srxn > 0) Entropy is a measure of the amount of disorder in a system—entropy must increase for any spontaneous change 1 Solutes and Solution The forces that drive the dissolution of a solute usually involve both enthalpy and entropy terms Hsoln < 0 for most species The creation of a solution takes a more ordered system (solid phase or pure liquid phase) and makes more disordered system (solute molecules are more randomly distributed throughout the solution) Saturation and Equilibrium If we have enough solute available, a solution can become saturated—the point when no more solute may be accepted into the solvent Saturation indicates an equilibrium between the pure solute and solvent and the solution solute + solvent solution KC 2 Saturation and Equilibrium solute + solvent solution KC The magnitude of KC indicates how soluble a solute is in that particular solvent If KC is large, the solute is very soluble If KC is small, the solute is only slightly soluble Saturation and Equilibrium Examples: + - NaCl(s) + H2O(l) Na (aq) + Cl (aq) KC = 37.3 A saturated solution of NaCl has a [Na+] = 6.11 M and [Cl-] = -

Weight and Lifestyle Inventory (Wali)
WEIGHT AND LIFESTYLE INVENTORY (Bariatric Surgery Version) © 2015 Thomas A. Wadden, Ph.D. and Gary D. Foster, Ph.D. 1 The Weight and Lifestyle Inventory (WALI) is designed to obtain information about your weight and dieting histories, your eating and exercise habits, and your relationships with family and friends. Please complete the questionnaire carefully and make your best guess when unsure of the answer. You will have an opportunity to review your answers with a member of our professional staff. Please allow 30-60 minutes to complete this questionnaire. Your answers will help us better identify problem areas and plan your treatment accordingly. The information you provide will become part of your medical record at Penn Medicine and may be shared with members of our treatment team. Thank you for taking the time to complete this questionnaire. SECTION A: IDENTIFYING INFORMATION ______________________________________________________________________________ 1 Name _________________________ __________ _______lbs. ________ft. ______inches 2 Date of Birth 3 Age 4 Weight 5 Height ______________________________________________________________________________ 6 Address ____________________ ________________________ ______________________/_______ yrs. 7 Phone: Cell 8 Phone: Home 9 Occupation/# of yrs. at job __________________________ 10 Today’s Date 11 Highest year of school completed: (Check one.) □ 6 □ 7 □ 8 □ 9 □ 10 □ 11 □ 12 □ 13 □ 14 □ 15 □ 16 □ Masters □ Doctorate Middle School High School College 12 Race (Check all that apply): □ American Indian □ Asian □ African American/Black □ Pacific Islander □White □ Other: ______________ 13 Are you Latino, Hispanic, or of Spanish origin? □ Yes □ No SECTION B: WEIGHT HISTORY 1. At what age were you first overweight by 10 lbs. or more? _______ yrs. old 2. What has been your highest weight after age 21? _______ lbs. -

Ideal Gas Law PV = Nrt R = Universal Gas Constant R = 0.08206 L-Atm R = 8.314 J Mol-K Mol-K Example
Ideal gas law PV = nRT R = universal gas constant R = 0.08206 L-atm R = 8.314 J mol-K mol-K Example: In the reaction of oxygen with carbon to produce carbon dioxide, how many liters of oxygen at 747 mm Hg and 21.0 °C are needed to react with 24.022 g of carbon? CHEM 102 Winter 2011 Practice: In the reaction of oxygen and hydrogen to make water, how many liters of hydrogen at exactly 0 °C and 1 atm are needed to react with 10.0 g of oxygen? 2 CHEM 102 Winter 2011 Combined gas law V decreases with pressure (V α 1/P). V increases with temperature (V α T). V increases with particles (V α n). Combining these ideas: V α n x T P For a given amount of gas (constant number of particles, ninitial = nafter) that changes in volume, temperature, or pressure: Vinitial α ninitial x Tinitial Pinitial Vafter α nafter x Tafter Pafter ninitial = n after Vinitial x Pinitial = Vafter x Pafter Tinitial Tafter 3 CHEM 102 Winter 2011 Example: A sample of gas is pressurized to 1.80 atm and volume of 90.0 cm3. What volume would the gas have if it were pressurized to 4.00 atm without a change in temperature? 4 CHEM 102 Winter 2011 Example: A 2547-mL sample of gas is at 421 °C. What is the new gas temperature if the gas is cooled at constant pressure until it has a volume of 1616 mL? 5 CHEM 102 Winter 2011 Practice: A balloon is filled with helium. -

Ideal Gasses Is Known As the Ideal Gas Law
ESCI 341 – Atmospheric Thermodynamics Lesson 4 –Ideal Gases References: An Introduction to Atmospheric Thermodynamics, Tsonis Introduction to Theoretical Meteorology, Hess Physical Chemistry (4th edition), Levine Thermodynamics and an Introduction to Thermostatistics, Callen IDEAL GASES An ideal gas is a gas with the following properties: There are no intermolecular forces, except during collisions. All collisions are elastic. The individual gas molecules have no volume (they behave like point masses). The equation of state for ideal gasses is known as the ideal gas law. The ideal gas law was discovered empirically, but can also be derived theoretically. The form we are most familiar with, pV nRT . Ideal Gas Law (1) R has a value of 8.3145 J-mol1-K1, and n is the number of moles (not molecules). A true ideal gas would be monatomic, meaning each molecule is comprised of a single atom. Real gasses in the atmosphere, such as O2 and N2, are diatomic, and some gasses such as CO2 and O3 are triatomic. Real atmospheric gasses have rotational and vibrational kinetic energy, in addition to translational kinetic energy. Even though the gasses that make up the atmosphere aren’t monatomic, they still closely obey the ideal gas law at the pressures and temperatures encountered in the atmosphere, so we can still use the ideal gas law. FORM OF IDEAL GAS LAW MOST USED BY METEOROLOGISTS In meteorology we use a modified form of the ideal gas law. We first divide (1) by volume to get n p RT . V we then multiply the RHS top and bottom by the molecular weight of the gas, M, to get Mn R p T . -

MAXIMUM METABOLISM FOOD METABOLISM PLAN the MAXIMUM Hreshold Spinach Salad: 1 Cup Spinach, 1/2 Cup Mixed 6 Oz
THE MAXIMUM METABOLISM FOOD PLAN™ WEEK ONE SUNDAY MONDAY TUESDAY WEDNESDAY THURSDAY FRIDAY SATURDAY Breakfast 3/4 oz. multi-grain cereal 1/2 cup oatmeal 2 lite pancakes (4” diameter); use non-stick 1 slice of wheat toast; top with 1/2 cup 1 1/2 oz. low fat granola Apple cinnamon crepe: 1 wheat tortilla, Omelet: 3 egg whites, 1 cup mixed zucchini, 1/2 sliced banana or 3/4 cup sliced 1/2 tbs. raisins pan; top with 1/2 cup peaches or 1/2 banana nonfat cottage cheese and 1/2 sliced 1 cup nonfat milk 1/3 cup nonfat ricotta cheese, 1 apple green peppers, onions, and tomatoes, 12 oz. of water before breakfast strawberries 1/2 cup nonfat milk 1 tbs. reduced calorie syrup apple; sprinkle with 1 tsp. cinnamon 1/2 medium banana (sliced), 1 tsp. cinnamon, 1/2 tsp. vanilla 2 oz. reduced calorie cheese, and salsa 1/2 cup nonfat milk 1 medium apple 1/2 cup low fat maple yogurt extract; heat in skillet over low flame 1 slice wheat toast 1/2 cup nonfat milk 1 tsp. butter Mid-Morning Snack 12 oz. water 12 oz. water 12 oz. water 12 oz. water 12 oz. water 12 oz. water 12 oz. water 1/2 bagel 1/2 cup nonfat yogurt 1/2 cup low fat cottage cheese 1 cup nonfat flavored yogurt with 1 tsp. 1 cup flavored nonfat yogurt 1 cup flavored nonfat yogurt 1 cup flavored nonfat yogurt 1 tbs. nonfat cream cheese 1/2 cup pineapple chunks sliced almonds 1 tbs. -

The Relation of Metabolic Rate to Body Weight and Organ Size
Pediat.Res. 1: 185-195 (1967) Metabolism basal metabolic rate growth REVIEW kidney glomerular filtration rate body surface area body weight oxygen consumption organ growth The Relation of Metabolic Rate to Body Weight and Organ Size A Review M.A. HOLLIDAY[54J, D. POTTER, A.JARRAH and S.BEARG Department of Pediatrics, University of California, San Francisco Medical Center, San Francisco, California, and the Children's Hospital Medical Center, Oakland, California, USA Introduction Historical Background The relation of metabolic rate to body size has been a The measurement of metabolic rate was first achieved subject of continuing interest to physicians, especially by LAVOISIER in 1780. By 1839 enough measurements pediatricians. It has been learned that many quanti- had been accumulated among subjects of different tative functions vary during growth in relation to sizes that it was suggested in a paper read before the metabolic rate, rather than body size. Examples of Royal Academy of France (co-authored by a professor these are cardiac output, glomerular filtration rate, of mathematics and a professor of medicine and oxygen consumption and drug dose. This phenomenon science) that BMR did not increase as body weight may reflect a direct cause and effect relation or may be increased but, rather, as surface area increased [42]. a fortuitous parallel between the relatively slower in- In 1889, RICHET [38] observed that BMR/kg in rab- crease in metabolic rate compared to body size and the bits of varying size decreased as body weight increased; function in question. RUBNER [41] made a similar observation in dogs. Both The fact that a decrease in metabolism and many noted that relating BMR to surface area provided re- other measures of physiological function in relation to a sults that did not vary significantly with size. -
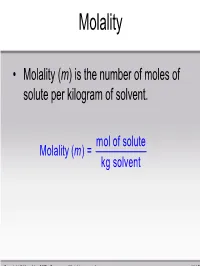
Mol of Solute Molality ( ) = Kg Solvent M
Molality • Molality (m) is the number of moles of solute per kilogram of solvent. mol of solute Molality (m ) = kg solvent Copyright © Houghton Mifflin Company. All rights reserved. 11 | 51 Sample Problem Calculate the molality of a solution of 13.5g of KF dissolved in 250. g of water. mol of solute m = kg solvent 1 m ol KF ()13.5g 58.1 = 0.250 kg = 0.929 m Copyright © Houghton Mifflin Company. All rights reserved. 11 | 52 Mole Fraction • Mole fraction (χ) is the ratio of the number of moles of a substance over the total number of moles of substances in solution. number of moles of i χ = i total number of moles ni = nT Copyright © Houghton Mifflin Company. All rights reserved. 11 | 53 Sample Problem -Conversions between units- • ex) What is the molality of a 0.200 M aluminum nitrate solution (d = 1.012g/mL)? – Work with 1 liter of solution. mass = 1012 g – mass Al(NO3)3 = 0.200 mol × 213.01 g/mol = 42.6 g ; – mass water = 1012 g -43 g = 969 g 0.200mol Molality==0.206 mol / kg 0.969kg Copyright © Houghton Mifflin Company. All rights reserved. 11 | 54 Sample Problem Calculate the mole fraction of 10.0g of NaCl dissolved in 100. g of water. mol of NaCl χ=NaCl mol of NaCl + mol H2 O 1 mol NaCl ()10.0g 58.5g NaCl = 1 m ol NaCl 1 m ol H2 O ()10.0g+ () 100.g H2 O 58.5g NaCl 18.0g H2 O = 0.0299 Copyright © Houghton Mifflin Company. -

Chapter 28 Pressure in Fluids
CHAPTER 28 PRESSURE IN FLUIDS EXERCISE 128, Page 281 1. A force of 280 N is applied to a piston of a hydraulic system of cross-sectional area 0.010 m 2 . Determine the pressure produced by the piston in the hydraulic fluid. force 280 N Pressure, p = = 28000Pa = 28 kPa area 0.010m2 That is, the pressure produced by the piston is 28 kPa 2. Find the force on the piston of question 1 to produce a pressure of 450 kPa. Pressure, p = 450 kPa = 450000 Pa force Pressure p = hence, force = pressure × area area = 4540000 × 0.010 = 4500 N = 4.5 kN 3. If the area of the piston in question 1 is halved and the force applied is 280 N, determine the new pressure in the hydraulic fluid. 0.010 New area = 0.005m2 2 force 280 N New pressure, p = = 56000Pa = 56 kPa area 0.005m2 331 © John Bird Published by Taylor and Francis EXERCISE 129, Page 283 1. Determine the pressure acting at the base of a dam, when the surface of the water is 35 m above base level. Take the density of water as 1000 kg/m 3 . Take the gravitational acceleration as 9.8 m/s 2 . Pressure at base of dam, p = gh = 1000 kg/m 3 9.8 m/s2 0.35 m = 343000 Pa = 343 kPa 2. An uncorked bottle is full of sea water of density 1030 kg/m 3 . Calculate, correct to 3 significant figures, the pressures on the side wall of the bottle at depths of (a) 30 mm, and (b) 70 mm below the top of the bottle. -

11 Archimedes Principle
Exp 11-1 P Experiment 11. Archimedes' Principle An application of Archimedes' principle - a piece of iron - sinks while a steel cup of the same weight floats on water. Objective: (a) To determine the specific gravity of a liquid by using a hydrometer. (b) the specific gravity of a solid heavier than water, the specific gravity of a liquid and the specific gravity of a solid lighter than water by· apj51yThg___Arcn1medes' principle: -- ····------···· --·-···- Apparatus: A balance, a solid heavier than water, a wooden cylinder, a hydrometer, alcohol and distilled water Theory: According to Archimedes' principle, when a body is immersed in a fluid (Fig. 1 ), either wholly or partially, the fluid exerts a buoyant force 8 on it. This buoyant force is equal to the weight of the fluid displaced by the body. Thus the apparent weight of the body is less than its actual weight, the loss of weight being equal to the buoyant force. - W = weight of the body B = buoyant force T = tension in the string supporting the body Fig. 1 Fig. 2 Consider the following problem: Weight of the body in air (actual weight of the body), w = 600 gm-wt Volume of the body, V = 80 cm3 Density of the liquid, p = 1.2 gm/cm 3 Volume of the liquid displaced = volume of the bodyVe = 80 cm3 Weight of the liquid displaced, we= Vex p = 96 gm-wt Buoyant force, B = 96 gm-wt Apparent weight of the body, wa = 600 - 96 gm-wt = 504 gm-wt Note that the apparent loss of weight of the body is equal to the - buoyant force of the fluid acting on the body, which, in turn, is equal to Exp 11-2P the weight of the flui9 displaced by the body. -

Molar Gas Constant
Molar gas constant The molar gas constant (also known as the universal or ideal gas constant and designated by the symbol R ) is an important physical constant which appears in many of the fundamental equations inphysics, chemistry and engineering, such as the ideal gas law, other equations of state and the Nernst equation. R is equivalent to the Boltzmann constant (designated kB) times Avogadro's constant (designated NA) and thus: R = kBNA. Currently the most accurate value as published by the National Institute of Standards and Technology (NIST) is:[1] R = 8.3144621 J · K–1 · mol–1 A number of values of R in other units are provided in the adjacent table. The gas constant occurs in the ideal gas law as follows: PV = nRT or as: PVm = RT where: P is the absolute pressure of the gas T is the absolute temperature of the gas V is the volume the gas occupies n is the number of moles of gas Vm is the molar volume of the gas The specific gas constant The gas constant of a specific gas, as differentiated from the above universal molar gas constant which applies for any ideal gas, is designated by the symbol Rs and is equal to the molar gas constant divided by the molecular mass (M ) of the gas: Rs = R ÷ M The specific gas constant for an ideal gas may also be obtained from the following thermodynamicsrelationship:[2] Rs = cp – cv where cp and cv are the gas's specific heats at constant pressure and constant volume respectively. Some example values of the specific gas constant are: –1 –1 –1 Ammonia (molecular mass of 17.032 g · mol ) : Rs = 0.4882 J · K · g –1 –1 –1 Hydrogen (molecular mass of 2.016 g · mol ) : Rs = 4.1242 J · K · g –1 –1 –1 Methane (molecular mass of 16.043 g · mol ) : Rs = 0.5183 J · K · g Unfortunately, many authors in the technical literature sometimes use R as the specific gas constant without designating it as such or stating that it is the specific gas constant. -

Thermodynamic Temperature
Thermodynamic temperature Thermodynamic temperature is the absolute measure 1 Overview of temperature and is one of the principal parameters of thermodynamics. Temperature is a measure of the random submicroscopic Thermodynamic temperature is defined by the third law motions and vibrations of the particle constituents of of thermodynamics in which the theoretically lowest tem- matter. These motions comprise the internal energy of perature is the null or zero point. At this point, absolute a substance. More specifically, the thermodynamic tem- zero, the particle constituents of matter have minimal perature of any bulk quantity of matter is the measure motion and can become no colder.[1][2] In the quantum- of the average kinetic energy per classical (i.e., non- mechanical description, matter at absolute zero is in its quantum) degree of freedom of its constituent particles. ground state, which is its state of lowest energy. Thermo- “Translational motions” are almost always in the classical dynamic temperature is often also called absolute tem- regime. Translational motions are ordinary, whole-body perature, for two reasons: one, proposed by Kelvin, that movements in three-dimensional space in which particles it does not depend on the properties of a particular mate- move about and exchange energy in collisions. Figure 1 rial; two that it refers to an absolute zero according to the below shows translational motion in gases; Figure 4 be- properties of the ideal gas. low shows translational motion in solids. Thermodynamic temperature’s null point, absolute zero, is the temperature The International System of Units specifies a particular at which the particle constituents of matter are as close as scale for thermodynamic temperature.