Molal Freezing Point Depression Constants SCIENTIFIC Calculating Kf Values
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Solutes and Solution
Solutes and Solution The first rule of solubility is “likes dissolve likes” Polar or ionic substances are soluble in polar solvents Non-polar substances are soluble in non- polar solvents Solutes and Solution There must be a reason why a substance is soluble in a solvent: either the solution process lowers the overall enthalpy of the system (Hrxn < 0) Or the solution process increases the overall entropy of the system (Srxn > 0) Entropy is a measure of the amount of disorder in a system—entropy must increase for any spontaneous change 1 Solutes and Solution The forces that drive the dissolution of a solute usually involve both enthalpy and entropy terms Hsoln < 0 for most species The creation of a solution takes a more ordered system (solid phase or pure liquid phase) and makes more disordered system (solute molecules are more randomly distributed throughout the solution) Saturation and Equilibrium If we have enough solute available, a solution can become saturated—the point when no more solute may be accepted into the solvent Saturation indicates an equilibrium between the pure solute and solvent and the solution solute + solvent solution KC 2 Saturation and Equilibrium solute + solvent solution KC The magnitude of KC indicates how soluble a solute is in that particular solvent If KC is large, the solute is very soluble If KC is small, the solute is only slightly soluble Saturation and Equilibrium Examples: + - NaCl(s) + H2O(l) Na (aq) + Cl (aq) KC = 37.3 A saturated solution of NaCl has a [Na+] = 6.11 M and [Cl-] = -

Fish Technology Glossary
Glossary of Fish Technology Terms A Selection of Terms Compiled by Kevin J. Whittle and Peter Howgate Prepared under contract to the Fisheries Industries Division of the Food and Agriculture Organization of the United Nations 6 December 2000 Last updated: February 2002 Kevin J. Whittle 1 GLOSSARY OF FISH TECHNOLOGY TERMS [Words highlighted in bold in the text of an entry refer to another entry. Words in parenthesis are alternatives.] Abnormalities Attributes of the fish that are not found in the great majority of that kind of fish. For example: atypical shapes; overall or patchy discolorations of skin or of fillet; diseased conditions; atypical odours or flavours. Generally, the term should be used for peculiarities present in the fish at the time of capture or harvesting, or developing very soon after; peculiarities arising during processing should be considered as defects. Acetic acid Formal chemical name, ethanoic acid. An organic acid of formula CH3.COOH. It is the main component, 3-6%, other than water, of vinegar. Used in fish technology in preparation of marinades. Acid curing See Marinating Actomyosin A combination of the two main proteins, actin and myosin, present in all muscle tissues. Additive A chemical added to a food to affect its properties. Objectives of including additives in a product include: increased stability during storage; inhibition of growth of microorganisms or production of microbial toxins; prevention or reduction of formation of off-flavours; improved sensory properties, particularly colours and appearance, affecting acceptability to the consumer; improved properties related to preparation and processing of food, for example, ability to create stable foams or emulsions, or to stabilise or thicken sauces. -

Δtb = M × Kb, Δtf = M × Kf
8.1HW Colligative Properties.doc Colligative Properties of Solvents Use the Equations given in your notes to solve the Colligative Property Questions. ΔTb = m × Kb, ΔTf = m × Kf Freezing Boiling K K Solvent Formula Point f b Point (°C) (°C/m) (°C/m) (°C) Water H2O 0.000 100.000 1.858 0.521 Acetic acid HC2H3O2 16.60 118.5 3.59 3.08 Benzene C6H6 5.455 80.2 5.065 2.61 Camphor C10H16O 179.5 ... 40 ... Carbon disulfide CS2 ... 46.3 ... 2.40 Cyclohexane C6H12 6.55 80.74 20.0 2.79 Ethanol C2H5OH ... 78.3 ... 1.07 1. Which solvent’s freezing point is depressed the most by the addition of a solute? This is determined by the Freezing Point Depression constant, Kf. The substance with the highest value for Kf will be affected the most. This would be Camphor with a constant of 40. 2. Which solvent’s freezing point is depressed the least by the addition of a solute? By the same logic as above, the substance with the lowest value for Kf will be affected the least. This is water. Certainly the case could be made that Carbon disulfide and Ethanol are affected the least as they do not have a constant. 3. Which solvent’s boiling point is elevated the least by the addition of a solute? Water 4. Which solvent’s boiling point is elevated the most by the addition of a solute? Acetic Acid 5. How does Kf relate to Kb? Kf > Kb (fill in the blank) The freezing point constant is always greater. -

Ideal Gas Law PV = Nrt R = Universal Gas Constant R = 0.08206 L-Atm R = 8.314 J Mol-K Mol-K Example
Ideal gas law PV = nRT R = universal gas constant R = 0.08206 L-atm R = 8.314 J mol-K mol-K Example: In the reaction of oxygen with carbon to produce carbon dioxide, how many liters of oxygen at 747 mm Hg and 21.0 °C are needed to react with 24.022 g of carbon? CHEM 102 Winter 2011 Practice: In the reaction of oxygen and hydrogen to make water, how many liters of hydrogen at exactly 0 °C and 1 atm are needed to react with 10.0 g of oxygen? 2 CHEM 102 Winter 2011 Combined gas law V decreases with pressure (V α 1/P). V increases with temperature (V α T). V increases with particles (V α n). Combining these ideas: V α n x T P For a given amount of gas (constant number of particles, ninitial = nafter) that changes in volume, temperature, or pressure: Vinitial α ninitial x Tinitial Pinitial Vafter α nafter x Tafter Pafter ninitial = n after Vinitial x Pinitial = Vafter x Pafter Tinitial Tafter 3 CHEM 102 Winter 2011 Example: A sample of gas is pressurized to 1.80 atm and volume of 90.0 cm3. What volume would the gas have if it were pressurized to 4.00 atm without a change in temperature? 4 CHEM 102 Winter 2011 Example: A 2547-mL sample of gas is at 421 °C. What is the new gas temperature if the gas is cooled at constant pressure until it has a volume of 1616 mL? 5 CHEM 102 Winter 2011 Practice: A balloon is filled with helium. -

Disrupting Crystalline Order to Restore Superfluidity
Abteilung Kommunikation und Öffentlichkeitsarbeit Referat Medien- und Öffentlichkeitsarbeit Tel. +49 40 42838-2968 Fax +49 40 42838-2449 E-Mail: [email protected] 12. Oktober 2018 Pressedienst 57/18 Disrupting crystalline order to restore superfluidity When we put water in a freezer, water molecules crystallize and form ice. This change from one phase of matter to another is called a phase transition. While this transition, and countless others that occur in nature, typically takes place at the same fixed conditions, such as the freezing point, one can ask how it can be influenced in a controlled way. We are all familiar with such control of the freezing transition, as it is an essential ingredient in the art of making a sorbet or a slushy. To make a cold and refreshing slushy with the perfect consistency, constant mixing of the liquid is needed. For example, a slush machine with constantly rotating blades helps prevent water molecules from crystalizing and turning the slushy into a solid block of ice. Imagine now controlling quantum matter in this same way. Rather than forming a normal liquid, like a melted slushy under the sun for too long, quantum matter can form a superfluid. This mysterious and counterintuitive form of matter was first observed in liquid helium at very low temperatures, less than 2 Kelvin above absolute zero. The helium atoms have a strong tendency to form a crystal, like the water molecules in a slushy, and this restricts the superfluid state of helium to very low temperatures and low pressures. But what if you could turn on the blades in your slush machine for quantum matter? What if you could disrupt the crystalline order so that the superfluid could flow freely even at temperatures and pressures where it usually does not? This is indeed the idea that was demonstrated by a team of scientists led by Ludwig Mathey and Andreas Hemmerich from the University of Hamburg. -

Physical Changes
How Matter Changes By Cindy Grigg Changes in matter happen around you every day. Some changes make matter look different. Other changes make one kind of matter become another kind of matter. When you scrunch a sheet of paper up into a ball, it is still paper. It only changed shape. You can cut a large, rectangular piece of paper into many small triangles. It changed shape and size, but it is still paper. These kinds of changes are called physical changes. Physical changes are changes in the way matter looks. Changes in size and shape, like the changes in the cut pieces of paper, are physical changes. Physical changes are changes in the size, shape, state, or appearance of matter. Another kind of physical change happens when matter changes from one state to another state. When water freezes and makes ice, it is still water. It has only changed its state of matter from a liquid to a solid. It has changed its appearance and shape, but it is still water. You can change the ice back into water by letting it melt. Matter looks different when it changes states, but it stays the same kind of matter. Solids like ice can change into liquids. Heat speeds up the moving particles in ice. The particles move apart. Heat melts ice and changes it to liquid water. Metals can be changed from a solid to a liquid state also. Metals must be heated to a high temperature to melt. Melting is changing from a solid state to a liquid state. -

Ideal Gasses Is Known As the Ideal Gas Law
ESCI 341 – Atmospheric Thermodynamics Lesson 4 –Ideal Gases References: An Introduction to Atmospheric Thermodynamics, Tsonis Introduction to Theoretical Meteorology, Hess Physical Chemistry (4th edition), Levine Thermodynamics and an Introduction to Thermostatistics, Callen IDEAL GASES An ideal gas is a gas with the following properties: There are no intermolecular forces, except during collisions. All collisions are elastic. The individual gas molecules have no volume (they behave like point masses). The equation of state for ideal gasses is known as the ideal gas law. The ideal gas law was discovered empirically, but can also be derived theoretically. The form we are most familiar with, pV nRT . Ideal Gas Law (1) R has a value of 8.3145 J-mol1-K1, and n is the number of moles (not molecules). A true ideal gas would be monatomic, meaning each molecule is comprised of a single atom. Real gasses in the atmosphere, such as O2 and N2, are diatomic, and some gasses such as CO2 and O3 are triatomic. Real atmospheric gasses have rotational and vibrational kinetic energy, in addition to translational kinetic energy. Even though the gasses that make up the atmosphere aren’t monatomic, they still closely obey the ideal gas law at the pressures and temperatures encountered in the atmosphere, so we can still use the ideal gas law. FORM OF IDEAL GAS LAW MOST USED BY METEOROLOGISTS In meteorology we use a modified form of the ideal gas law. We first divide (1) by volume to get n p RT . V we then multiply the RHS top and bottom by the molecular weight of the gas, M, to get Mn R p T . -

A Possible Force Mechanism Associated with the Freezing of Water in Porous Materials
A Possible Force Mechanism Associated with the Freezing of Water in Porous Materials LORNE W. GOLD Division of Building Research, National Research Council, Ottawa, Canada The thermodynamic equilibrium conditions for a water-ice interface in a pore of a porous medium are derived. It is found that, taking the geometry and physical dimensions of the pore into consideration, a positive pressure must develop between the ice and the solid in order for equilibrium to be maintained when the temperature at the freezing plane is depressed. A simple model utilizing this mecha• nism exhibits the same properties as a frost-heaving soil. The in• fluence of air in the pores is briefly discussed. #WHEN WATER is present in porous materials and is allowed to freeze, a force may be developed which is not directly related to the expansion that occurs when water changes to ice. Although many observations have been made on this phenomenon, par• ticularly in the field of soil mechanics, a satisfactory explanation of the origin of this force has not appeared. Experiments to date indicate how the magnitude of this force depends on certain physical characteristics of the system. The following are some of the facts observed during experiments that a theory on the origin of this force must explain: 1. During the freezing process, the freezing plane may remain stationary while the ice phase continues to grow. When this occurs, the frozen portion is displaced rela• tive to the unfrozen and a layer of ice, often called an ice lens is formed. The dis• placement may be microscopic or it may amount to several inches. -
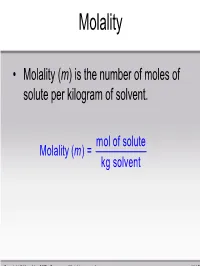
Mol of Solute Molality ( ) = Kg Solvent M
Molality • Molality (m) is the number of moles of solute per kilogram of solvent. mol of solute Molality (m ) = kg solvent Copyright © Houghton Mifflin Company. All rights reserved. 11 | 51 Sample Problem Calculate the molality of a solution of 13.5g of KF dissolved in 250. g of water. mol of solute m = kg solvent 1 m ol KF ()13.5g 58.1 = 0.250 kg = 0.929 m Copyright © Houghton Mifflin Company. All rights reserved. 11 | 52 Mole Fraction • Mole fraction (χ) is the ratio of the number of moles of a substance over the total number of moles of substances in solution. number of moles of i χ = i total number of moles ni = nT Copyright © Houghton Mifflin Company. All rights reserved. 11 | 53 Sample Problem -Conversions between units- • ex) What is the molality of a 0.200 M aluminum nitrate solution (d = 1.012g/mL)? – Work with 1 liter of solution. mass = 1012 g – mass Al(NO3)3 = 0.200 mol × 213.01 g/mol = 42.6 g ; – mass water = 1012 g -43 g = 969 g 0.200mol Molality==0.206 mol / kg 0.969kg Copyright © Houghton Mifflin Company. All rights reserved. 11 | 54 Sample Problem Calculate the mole fraction of 10.0g of NaCl dissolved in 100. g of water. mol of NaCl χ=NaCl mol of NaCl + mol H2 O 1 mol NaCl ()10.0g 58.5g NaCl = 1 m ol NaCl 1 m ol H2 O ()10.0g+ () 100.g H2 O 58.5g NaCl 18.0g H2 O = 0.0299 Copyright © Houghton Mifflin Company. -

Understanding Freezing Behavior in Nano-Porous Materials: Free
ÍÒÖ×ØÒÒ ÖÞÒ ÚÓÖ Ò ÆÒÓ¹Ô ÓÖÓÙ× ÅØÖÐ× Ö ÒÖ Ý ËÑÙÐØÓÒ ËØÙ× Ò ÜÔ ÖÑÒØ ××ÖØØÓÒ ÈÖ×ÒØ ØÓ Ø ÙÐØÝ Ó Ø Ö ÙØ Ë Ó ÓÐ Ó ÓÖÒÐÐ ÍÒÚÖ×ØÝ Ò ÈÖØÐ ÙЬÐÐÑÒØ Ó Ø ÊÕÙÖÑÒØ× ÓÖ Ø Ö Ó Ó ØÓÖ Ó È ÐÓ×ÓÔ Ý Ý ÊÚ ÊÖ×ÒÒ ÙÙ×Ø ¾¼¼¼ ÊÚ ÊÖ×ÒÒ ¾¼¼¼ ÄÄ ÊÁÀÌË ÊËÊÎ ÍÒÖ×ØÒÒ ÖÞÒ ÚÓÖ Ò ÆÒÓ¹Ô ÓÖÓÙ× ÅØÖÐ× Ö ÒÖÝ ËÑÙÐØÓÒ ËØÙ× Ò ÜÔ ÖÑÒØ ÊÚ ÊÖ×ÒÒ¸ Ⱥº ÓÖÒÐÐ ÍÒÚÖ×ØÝ ¾¼¼¼ ÍÒÖ×ØÒÒ Ô× ÚÓÖ Ò ÓÒ¬Ò ×Ý×ØÑ× × ØÖÑÒÓÙ× Ô ÓØÒØÐ Ò ÑÔÖÓÚÒ Ø Æ ÓÚÖØÝ Ó ×ÔÖØÓÒ ÔÖÓ ××× ØØ Ù× Ô ÓÖÓÙ× Ñ¹ ØÖÐ× Ð ØÚØ Ö ÓÒ׸ ÓÒØÖÓÐÐ Ð××׸ ×Ð ÜÖÓÐ× ² ÖÓÐ׸ Ö ÓÒ ÖÓÐ× Ò ÞÓÐØ׺ Ì «Ø× Ó Ø ÖÙ ÑÒ×ÓÒÐØÝ Ò ÒÒ ÒÖ¹ Ø ÒØÖØÓÒ× Ù ØÓ Ø ÔÓÖÓÙ× ×ÙÖ Ú ÑÔ ÓÖØÒØ ÓÒ×ÕÙÒ× ØØ Ò ÓÒÐÝ ÔØÙÖ Ý ÑÓÐÙÐÖ ÐÚÐ ÑÓ ÐÒº ÆÓÚÐ Ô× ØÖÒ×ØÓÒ× Ò Ö×ÙÐØ ´ÔÐÐÖÝ ÓÒÒ×ØÓÒ Ò ÕÙ×¹ÓÒ¹ÑÒ×ÓÒÐ ×Ý×ØÑ× Ò ÓÖÒØØÓÒÐ ÓÖÖÒ ØÖÒ×ØÓÒ× Ò ÕÙ×¹ØÛÓ¹ÑÒ×ÓÒÐ ×Ý×ØÑ×µ ØØ ÓØÒ Ö Ø Ù× Ó Ø Ö¹ ÓÛÒ Ó ÑÖÓ×ÓÔ ÕÙØÓÒ× Ð ÃÐÚÒ Ò ×¹ÌÓÑ×ÓÒ ÕÙØÓÒ× × ÓÒ Ð××Ð ØÖÑÓ ÝÒÑ׺ ÁÒ Ø× ÛÓÖ¸ Ø Ó Ù× ÓÒ Ö ÒÖÝ ÑØÓ × ÓÒ ÄÒÙ ØÓÖÝ Û Û ÚÐÓÔ Ò ÔÔÐØÓÒ× Ó Ø× ØÓÖÝ ØÓ ÙÒÖ×ØÒ Ø ÖÓÛÒ Ó Ø ÑÖÓ×ÓÔ ÕÙØÓÒ׸ ÚÐÓÔÒ ÐÓÐ Ô× ÖÑ׸ Ò ÖØÖÞÒ ÜØ Ô×׺ Ì Ö×ÙÐØ× Ó ÓÙÖ ÜÔ ÖÑÒØÐ ×ØÙ× Ö Ð×Ó ×Ö ¸ ØØ ÔÖÓÚ ×ÙÔÔ ÓÖØÒ ÚÒ ÓÖ ÓÙÖ ÑÓ ÐÒ «ÓÖØ׺ ÓÖÔÐ Ë Ø ÓÖÒ Ò Ò Ñ ÑÐÝ Ò ×ÓÙØÖÒ ÁÒ¸ Á Û× ÒØÖ×Ø Ò Ö×Ö ×Ò Ø Ý× Á Ò³Ø ÚÒ ÖÑÑÖº Á ÓÙÒ ÑÝ×Ð ´ÖØÖ ÓÖØÙÒØÐÝ ÓÖ Ñµ ×ØÙÝÒ ÑÐ ÒÒÖÒ Ò Ø ÁÒÒ ÁÒ×ØØÙØ Ó ÌÒÓÐÓÝ Ø ÅÖ׺ ÙÖÒ ÑÝ Ö×ÑÒ ÝÖ Á Û× ×ØÖÙ Ý Ø ÖÐÞØÓÒ ØØ Á Ñ ÒÓ ÐÓÒÖ Ø ÒÓÛ¹ÐÐ Ô Ö×ÓÒ Ò ×Ó Óи ØØ Ø ÛÓÖÐ × ÙÐÐ Ó ÔÝ×Ð ´Ò Ñе ÔÒÓÑÒ ØØ Á ÖÐÝ ÒÛ ÓÙغ Ø Ø ÒÒÒ Ó ÑÝÂÙÒÓÖÝÖ¸ Á ×ØÖØ ØÓ ÚÐÓÔ Ò ÒØÒ× ×ÒØÓÒ ÓÖ ×ØØ×ØÐ ÑÒ× Ò Ò ÑÝ ÑÒ ØÓ ÕÙØ ÑÐ ÒÒÖÒ Ò Ø ÙÔ ÔÝ×׺ ÁØ Û× Ô× ÛÖ Á Û× ÑÒ Ø ÓÒØÒØ× Ó ÑÒÝ Ó Ó× ÓÒ ×ØØ×ØÐ ÔÝ×׸ ÛÒ Á Ñ ÖÓ×× Ø ÓÓ ÅÓÐÙÐÖ ÌÖÑÓ ÝÒÑ× Ó ÐÙ È× ÕÙÐÖ Ý ÂÓÒ ÈÖÙ×ÒØÞº Ì× Û× ØÙÖÒÒ -

Freezing Convenience Foods Include: • You Prepare Food When You Have Time
FreezingFreezing convenienceconvenience foodsfoods that you’ve prepared at home PNW 296 By C. Raab and N. Oehler our freezer can help you prepare for busy days • You can save money by making convenience foods ahead, parties, or unexpected company. By yourself. planning a steady flow of main dishes, baked Y On the other hand: goods, desserts, and other foods, you can make good • Freezing is expensive when you total the cost of use of your freezer and your time. packaging, energy use, and the freezer itself. Benefits of freezing convenience foods include: • You prepare food when you have time. • You use more energy to cook, freeze, and reheat a dish than you would use to cook it for immediate • You use your oven more efficiently by baking more consumption. than one dish at a time. • Prepared foods have a relatively short storage life • You avoid waste by freezing leftovers to use as compared to the storage life of their ingredients “planned overs.” (such as frozen fruits, vegetables, and meat). • You can prepare special diet foods and baby foods in • Unless you have a microwave, you must allow quantity and freeze them in single portions. plenty of time for thawing. • You save time by doubling or tripling recipes and • Some products don’t freeze well. Others don’t justify freezing the extra food. the labor and expense of freezing. • If you normally cook for just one or two, you can freeze individual portions of an ordinary recipe for later use. A Pacific Northwest Extension Publication Oregon State University • Washington State University • University of Idaho Contents • Use moisture-vapor-resistant packaging such as plas- Preparing foods for freezing .......................................2 tic containers, freezer bags, heavy-duty aluminum Freezer storage ............................................................2 foil, and coated freezer paper to preserve the quality From the freezer to the table .......................................3 of frozen food. -

Molar Gas Constant
Molar gas constant The molar gas constant (also known as the universal or ideal gas constant and designated by the symbol R ) is an important physical constant which appears in many of the fundamental equations inphysics, chemistry and engineering, such as the ideal gas law, other equations of state and the Nernst equation. R is equivalent to the Boltzmann constant (designated kB) times Avogadro's constant (designated NA) and thus: R = kBNA. Currently the most accurate value as published by the National Institute of Standards and Technology (NIST) is:[1] R = 8.3144621 J · K–1 · mol–1 A number of values of R in other units are provided in the adjacent table. The gas constant occurs in the ideal gas law as follows: PV = nRT or as: PVm = RT where: P is the absolute pressure of the gas T is the absolute temperature of the gas V is the volume the gas occupies n is the number of moles of gas Vm is the molar volume of the gas The specific gas constant The gas constant of a specific gas, as differentiated from the above universal molar gas constant which applies for any ideal gas, is designated by the symbol Rs and is equal to the molar gas constant divided by the molecular mass (M ) of the gas: Rs = R ÷ M The specific gas constant for an ideal gas may also be obtained from the following thermodynamicsrelationship:[2] Rs = cp – cv where cp and cv are the gas's specific heats at constant pressure and constant volume respectively. Some example values of the specific gas constant are: –1 –1 –1 Ammonia (molecular mass of 17.032 g · mol ) : Rs = 0.4882 J · K · g –1 –1 –1 Hydrogen (molecular mass of 2.016 g · mol ) : Rs = 4.1242 J · K · g –1 –1 –1 Methane (molecular mass of 16.043 g · mol ) : Rs = 0.5183 J · K · g Unfortunately, many authors in the technical literature sometimes use R as the specific gas constant without designating it as such or stating that it is the specific gas constant.