Ideal Gas Law
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Solutes and Solution
Solutes and Solution The first rule of solubility is “likes dissolve likes” Polar or ionic substances are soluble in polar solvents Non-polar substances are soluble in non- polar solvents Solutes and Solution There must be a reason why a substance is soluble in a solvent: either the solution process lowers the overall enthalpy of the system (Hrxn < 0) Or the solution process increases the overall entropy of the system (Srxn > 0) Entropy is a measure of the amount of disorder in a system—entropy must increase for any spontaneous change 1 Solutes and Solution The forces that drive the dissolution of a solute usually involve both enthalpy and entropy terms Hsoln < 0 for most species The creation of a solution takes a more ordered system (solid phase or pure liquid phase) and makes more disordered system (solute molecules are more randomly distributed throughout the solution) Saturation and Equilibrium If we have enough solute available, a solution can become saturated—the point when no more solute may be accepted into the solvent Saturation indicates an equilibrium between the pure solute and solvent and the solution solute + solvent solution KC 2 Saturation and Equilibrium solute + solvent solution KC The magnitude of KC indicates how soluble a solute is in that particular solvent If KC is large, the solute is very soluble If KC is small, the solute is only slightly soluble Saturation and Equilibrium Examples: + - NaCl(s) + H2O(l) Na (aq) + Cl (aq) KC = 37.3 A saturated solution of NaCl has a [Na+] = 6.11 M and [Cl-] = -

Entropy: Ideal Gas Processes
Chapter 19: The Kinec Theory of Gases Thermodynamics = macroscopic picture Gases micro -> macro picture One mole is the number of atoms in 12 g sample Avogadro’s Number of carbon-12 23 -1 C(12)—6 protrons, 6 neutrons and 6 electrons NA=6.02 x 10 mol 12 atomic units of mass assuming mP=mn Another way to do this is to know the mass of one molecule: then So the number of moles n is given by M n=N/N sample A N = N A mmole−mass € Ideal Gas Law Ideal Gases, Ideal Gas Law It was found experimentally that if 1 mole of any gas is placed in containers that have the same volume V and are kept at the same temperature T, approximately all have the same pressure p. The small differences in pressure disappear if lower gas densities are used. Further experiments showed that all low-density gases obey the equation pV = nRT. Here R = 8.31 K/mol ⋅ K and is known as the "gas constant." The equation itself is known as the "ideal gas law." The constant R can be expressed -23 as R = kNA . Here k is called the Boltzmann constant and is equal to 1.38 × 10 J/K. N If we substitute R as well as n = in the ideal gas law we get the equivalent form: NA pV = NkT. Here N is the number of molecules in the gas. The behavior of all real gases approaches that of an ideal gas at low enough densities. Low densitiens m= enumberans tha oft t hemoles gas molecul es are fa Nr e=nough number apa ofr tparticles that the y do not interact with one another, but only with the walls of the gas container. -

IB Questionbank
Topic 3 Past Paper [94 marks] This question is about thermal energy transfer. A hot piece of iron is placed into a container of cold water. After a time the iron and water reach thermal equilibrium. The heat capacity of the container is negligible. specific heat capacity. [2 marks] 1a. Define Markscheme the energy required to change the temperature (of a substance) by 1K/°C/unit degree; of mass 1 kg / per unit mass; [5 marks] 1b. The following data are available. Mass of water = 0.35 kg Mass of iron = 0.58 kg Specific heat capacity of water = 4200 J kg–1K–1 Initial temperature of water = 20°C Final temperature of water = 44°C Initial temperature of iron = 180°C (i) Determine the specific heat capacity of iron. (ii) Explain why the value calculated in (b)(i) is likely to be different from the accepted value. Markscheme (i) use of mcΔT; 0.58×c×[180-44]=0.35×4200×[44-20]; c=447Jkg-1K-1≈450Jkg-1K-1; (ii) energy would be given off to surroundings/environment / energy would be absorbed by container / energy would be given off through vaporization of water; hence final temperature would be less; hence measured value of (specific) heat capacity (of iron) would be higher; This question is in two parts. Part 1 is about ideal gases and specific heat capacity. Part 2 is about simple harmonic motion and waves. Part 1 Ideal gases and specific heat capacity State assumptions of the kinetic model of an ideal gas. [2 marks] 2a. two Markscheme point molecules / negligible volume; no forces between molecules except during contact; motion/distribution is random; elastic collisions / no energy lost; obey Newton’s laws of motion; collision in zero time; gravity is ignored; [4 marks] 2b. -

Thermodynamics of Ideal Gases
D Thermodynamics of ideal gases An ideal gas is a nice “laboratory” for understanding the thermodynamics of a fluid with a non-trivial equation of state. In this section we shall recapitulate the conventional thermodynamics of an ideal gas with constant heat capacity. For more extensive treatments, see for example [67, 66]. D.1 Internal energy In section 4.1 we analyzed Bernoulli’s model of a gas consisting of essentially 1 2 non-interacting point-like molecules, and found the pressure p = 3 ½ v where v is the root-mean-square average molecular speed. Using the ideal gas law (4-26) the total molecular kinetic energy contained in an amount M = ½V of the gas becomes, 1 3 3 Mv2 = pV = nRT ; (D-1) 2 2 2 where n = M=Mmol is the number of moles in the gas. The derivation in section 4.1 shows that the factor 3 stems from the three independent translational degrees of freedom available to point-like molecules. The above formula thus expresses 1 that in a mole of a gas there is an internal kinetic energy 2 RT associated with each translational degree of freedom of the point-like molecules. Whereas monatomic gases like argon have spherical molecules and thus only the three translational degrees of freedom, diatomic gases like nitrogen and oxy- Copyright °c 1998{2004, Benny Lautrup Revision 7.7, January 22, 2004 662 D. THERMODYNAMICS OF IDEAL GASES gen have stick-like molecules with two extra rotational degrees of freedom or- thogonally to the bridge connecting the atoms, and multiatomic gases like carbon dioxide and methane have the three extra rotational degrees of freedom. -

Useful Constants
Appendix A Useful Constants A.1 Physical Constants Table A.1 Physical constants in SI units Symbol Constant Value c Speed of light 2.997925 × 108 m/s −19 e Elementary charge 1.602191 × 10 C −12 2 2 3 ε0 Permittivity 8.854 × 10 C s / kgm −7 2 μ0 Permeability 4π × 10 kgm/C −27 mH Atomic mass unit 1.660531 × 10 kg −31 me Electron mass 9.109558 × 10 kg −27 mp Proton mass 1.672614 × 10 kg −27 mn Neutron mass 1.674920 × 10 kg h Planck constant 6.626196 × 10−34 Js h¯ Planck constant 1.054591 × 10−34 Js R Gas constant 8.314510 × 103 J/(kgK) −23 k Boltzmann constant 1.380622 × 10 J/K −8 2 4 σ Stefan–Boltzmann constant 5.66961 × 10 W/ m K G Gravitational constant 6.6732 × 10−11 m3/ kgs2 M. Benacquista, An Introduction to the Evolution of Single and Binary Stars, 223 Undergraduate Lecture Notes in Physics, DOI 10.1007/978-1-4419-9991-7, © Springer Science+Business Media New York 2013 224 A Useful Constants Table A.2 Useful combinations and alternate units Symbol Constant Value 2 mHc Atomic mass unit 931.50MeV 2 mec Electron rest mass energy 511.00keV 2 mpc Proton rest mass energy 938.28MeV 2 mnc Neutron rest mass energy 939.57MeV h Planck constant 4.136 × 10−15 eVs h¯ Planck constant 6.582 × 10−16 eVs k Boltzmann constant 8.617 × 10−5 eV/K hc 1,240eVnm hc¯ 197.3eVnm 2 e /(4πε0) 1.440eVnm A.2 Astronomical Constants Table A.3 Astronomical units Symbol Constant Value AU Astronomical unit 1.4959787066 × 1011 m ly Light year 9.460730472 × 1015 m pc Parsec 2.0624806 × 105 AU 3.2615638ly 3.0856776 × 1016 m d Sidereal day 23h 56m 04.0905309s 8.61640905309 -

5.5 ENTROPY CHANGES of an IDEAL GAS for One Mole Or a Unit Mass of Fluid Undergoing a Mechanically Reversible Process in a Closed System, the First Law, Eq
Thermodynamic Third class Dr. Arkan J. Hadi 5.5 ENTROPY CHANGES OF AN IDEAL GAS For one mole or a unit mass of fluid undergoing a mechanically reversible process in a closed system, the first law, Eq. (2.8), becomes: Differentiation of the defining equation for enthalpy, H = U + PV, yields: Eliminating dU gives: For an ideal gas, dH = and V = RT/ P. With these substitutions and then division by T, As a result of Eq. (5.11), this becomes: where S is the molar entropy of an ideal gas. Integration from an initial state at conditions To and Po to a final state at conditions T and P gives: Although derived for a mechanically reversible process, this equation relates properties only, and is independent of the process causing the change of state. It is therefore a general equation for the calculation of entropy changes of an ideal gas. 1 Thermodynamic Third class Dr. Arkan J. Hadi 5.6 MATHEMATICAL STATEMENT OF THE SECOND LAW Consider two heat reservoirs, one at temperature TH and a second at the lower temperature Tc. Let a quantity of heat | | be transferred from the hotter to the cooler reservoir. The entropy changes of the reservoirs at TH and at Tc are: These two entropy changes are added to give: Since TH > Tc, the total entropy change as a result of this irreversible process is positive. Also, ΔStotal becomes smaller as the difference TH - TC gets smaller. When TH is only infinitesimally higher than Tc, the heat transfer is reversible, and ΔStotal approaches zero. Thus for the process of irreversible heat transfer, ΔStotal is always positive, approaching zero as the process becomes reversible. -

Ideal Gas Law PV = Nrt R = Universal Gas Constant R = 0.08206 L-Atm R = 8.314 J Mol-K Mol-K Example
Ideal gas law PV = nRT R = universal gas constant R = 0.08206 L-atm R = 8.314 J mol-K mol-K Example: In the reaction of oxygen with carbon to produce carbon dioxide, how many liters of oxygen at 747 mm Hg and 21.0 °C are needed to react with 24.022 g of carbon? CHEM 102 Winter 2011 Practice: In the reaction of oxygen and hydrogen to make water, how many liters of hydrogen at exactly 0 °C and 1 atm are needed to react with 10.0 g of oxygen? 2 CHEM 102 Winter 2011 Combined gas law V decreases with pressure (V α 1/P). V increases with temperature (V α T). V increases with particles (V α n). Combining these ideas: V α n x T P For a given amount of gas (constant number of particles, ninitial = nafter) that changes in volume, temperature, or pressure: Vinitial α ninitial x Tinitial Pinitial Vafter α nafter x Tafter Pafter ninitial = n after Vinitial x Pinitial = Vafter x Pafter Tinitial Tafter 3 CHEM 102 Winter 2011 Example: A sample of gas is pressurized to 1.80 atm and volume of 90.0 cm3. What volume would the gas have if it were pressurized to 4.00 atm without a change in temperature? 4 CHEM 102 Winter 2011 Example: A 2547-mL sample of gas is at 421 °C. What is the new gas temperature if the gas is cooled at constant pressure until it has a volume of 1616 mL? 5 CHEM 102 Winter 2011 Practice: A balloon is filled with helium. -

Chapter 3 3.4-2 the Compressibility Factor Equation of State
Chapter 3 3.4-2 The Compressibility Factor Equation of State The dimensionless compressibility factor, Z, for a gaseous species is defined as the ratio pv Z = (3.4-1) RT If the gas behaves ideally Z = 1. The extent to which Z differs from 1 is a measure of the extent to which the gas is behaving nonideally. The compressibility can be determined from experimental data where Z is plotted versus a dimensionless reduced pressure pR and reduced temperature TR, defined as pR = p/pc and TR = T/Tc In these expressions, pc and Tc denote the critical pressure and temperature, respectively. A generalized compressibility chart of the form Z = f(pR, TR) is shown in Figure 3.4-1 for 10 different gases. The solid lines represent the best curves fitted to the data. Figure 3.4-1 Generalized compressibility chart for various gases10. It can be seen from Figure 3.4-1 that the value of Z tends to unity for all temperatures as pressure approach zero and Z also approaches unity for all pressure at very high temperature. If the p, v, and T data are available in table format or computer software then you should not use the generalized compressibility chart to evaluate p, v, and T since using Z is just another approximation to the real data. 10 Moran, M. J. and Shapiro H. N., Fundamentals of Engineering Thermodynamics, Wiley, 2008, pg. 112 3-19 Example 3.4-2 ---------------------------------------------------------------------------------- A closed, rigid tank filled with water vapor, initially at 20 MPa, 520oC, is cooled until its temperature reaches 400oC. -

Ideal Gasses Is Known As the Ideal Gas Law
ESCI 341 – Atmospheric Thermodynamics Lesson 4 –Ideal Gases References: An Introduction to Atmospheric Thermodynamics, Tsonis Introduction to Theoretical Meteorology, Hess Physical Chemistry (4th edition), Levine Thermodynamics and an Introduction to Thermostatistics, Callen IDEAL GASES An ideal gas is a gas with the following properties: There are no intermolecular forces, except during collisions. All collisions are elastic. The individual gas molecules have no volume (they behave like point masses). The equation of state for ideal gasses is known as the ideal gas law. The ideal gas law was discovered empirically, but can also be derived theoretically. The form we are most familiar with, pV nRT . Ideal Gas Law (1) R has a value of 8.3145 J-mol1-K1, and n is the number of moles (not molecules). A true ideal gas would be monatomic, meaning each molecule is comprised of a single atom. Real gasses in the atmosphere, such as O2 and N2, are diatomic, and some gasses such as CO2 and O3 are triatomic. Real atmospheric gasses have rotational and vibrational kinetic energy, in addition to translational kinetic energy. Even though the gasses that make up the atmosphere aren’t monatomic, they still closely obey the ideal gas law at the pressures and temperatures encountered in the atmosphere, so we can still use the ideal gas law. FORM OF IDEAL GAS LAW MOST USED BY METEOROLOGISTS In meteorology we use a modified form of the ideal gas law. We first divide (1) by volume to get n p RT . V we then multiply the RHS top and bottom by the molecular weight of the gas, M, to get Mn R p T . -

The Discovery of Thermodynamics
Philosophical Magazine ISSN: 1478-6435 (Print) 1478-6443 (Online) Journal homepage: https://www.tandfonline.com/loi/tphm20 The discovery of thermodynamics Peter Weinberger To cite this article: Peter Weinberger (2013) The discovery of thermodynamics, Philosophical Magazine, 93:20, 2576-2612, DOI: 10.1080/14786435.2013.784402 To link to this article: https://doi.org/10.1080/14786435.2013.784402 Published online: 09 Apr 2013. Submit your article to this journal Article views: 658 Citing articles: 2 View citing articles Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=tphm20 Philosophical Magazine, 2013 Vol. 93, No. 20, 2576–2612, http://dx.doi.org/10.1080/14786435.2013.784402 COMMENTARY The discovery of thermodynamics Peter Weinberger∗ Center for Computational Nanoscience, Seilerstätte 10/21, A1010 Vienna, Austria (Received 21 December 2012; final version received 6 March 2013) Based on the idea that a scientific journal is also an “agora” (Greek: market place) for the exchange of ideas and scientific concepts, the history of thermodynamics between 1800 and 1910 as documented in the Philosophical Magazine Archives is uncovered. Famous scientists such as Joule, Thomson (Lord Kelvin), Clau- sius, Maxwell or Boltzmann shared this forum. Not always in the most friendly manner. It is interesting to find out, how difficult it was to describe in a scientific (mathematical) language a phenomenon like “heat”, to see, how long it took to arrive at one of the fundamental principles in physics: entropy. Scientific progress started from the simple rule of Boyle and Mariotte dating from the late eighteenth century and arrived in the twentieth century with the concept of probabilities. -

Module P7.4 Specific Heat, Latent Heat and Entropy
FLEXIBLE LEARNING APPROACH TO PHYSICS Module P7.4 Specific heat, latent heat and entropy 1 Opening items 4 PVT-surfaces and changes of phase 1.1 Module introduction 4.1 The critical point 1.2 Fast track questions 4.2 The triple point 1.3 Ready to study? 4.3 The Clausius–Clapeyron equation 2 Heating solids and liquids 5 Entropy and the second law of thermodynamics 2.1 Heat, work and internal energy 5.1 The second law of thermodynamics 2.2 Changes of temperature: specific heat 5.2 Entropy: a function of state 2.3 Changes of phase: latent heat 5.3 The principle of entropy increase 2.4 Measuring specific heats and latent heats 5.4 The irreversibility of nature 3 Heating gases 6 Closing items 3.1 Ideal gases 6.1 Module summary 3.2 Principal specific heats: monatomic ideal gases 6.2 Achievements 3.3 Principal specific heats: other gases 6.3 Exit test 3.4 Isothermal and adiabatic processes Exit module FLAP P7.4 Specific heat, latent heat and entropy COPYRIGHT © 1998 THE OPEN UNIVERSITY S570 V1.1 1 Opening items 1.1 Module introduction What happens when a substance is heated? Its temperature may rise; it may melt or evaporate; it may expand and do work1—1the net effect of the heating depends on the conditions under which the heating takes place. In this module we discuss the heating of solids, liquids and gases under a variety of conditions. We also look more generally at the problem of converting heat into useful work, and the related issue of the irreversibility of many natural processes. -
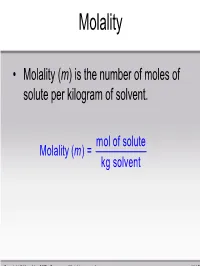
Mol of Solute Molality ( ) = Kg Solvent M
Molality • Molality (m) is the number of moles of solute per kilogram of solvent. mol of solute Molality (m ) = kg solvent Copyright © Houghton Mifflin Company. All rights reserved. 11 | 51 Sample Problem Calculate the molality of a solution of 13.5g of KF dissolved in 250. g of water. mol of solute m = kg solvent 1 m ol KF ()13.5g 58.1 = 0.250 kg = 0.929 m Copyright © Houghton Mifflin Company. All rights reserved. 11 | 52 Mole Fraction • Mole fraction (χ) is the ratio of the number of moles of a substance over the total number of moles of substances in solution. number of moles of i χ = i total number of moles ni = nT Copyright © Houghton Mifflin Company. All rights reserved. 11 | 53 Sample Problem -Conversions between units- • ex) What is the molality of a 0.200 M aluminum nitrate solution (d = 1.012g/mL)? – Work with 1 liter of solution. mass = 1012 g – mass Al(NO3)3 = 0.200 mol × 213.01 g/mol = 42.6 g ; – mass water = 1012 g -43 g = 969 g 0.200mol Molality==0.206 mol / kg 0.969kg Copyright © Houghton Mifflin Company. All rights reserved. 11 | 54 Sample Problem Calculate the mole fraction of 10.0g of NaCl dissolved in 100. g of water. mol of NaCl χ=NaCl mol of NaCl + mol H2 O 1 mol NaCl ()10.0g 58.5g NaCl = 1 m ol NaCl 1 m ol H2 O ()10.0g+ () 100.g H2 O 58.5g NaCl 18.0g H2 O = 0.0299 Copyright © Houghton Mifflin Company.