The Discovery of Thermodynamics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Entropy: Ideal Gas Processes
Chapter 19: The Kinec Theory of Gases Thermodynamics = macroscopic picture Gases micro -> macro picture One mole is the number of atoms in 12 g sample Avogadro’s Number of carbon-12 23 -1 C(12)—6 protrons, 6 neutrons and 6 electrons NA=6.02 x 10 mol 12 atomic units of mass assuming mP=mn Another way to do this is to know the mass of one molecule: then So the number of moles n is given by M n=N/N sample A N = N A mmole−mass € Ideal Gas Law Ideal Gases, Ideal Gas Law It was found experimentally that if 1 mole of any gas is placed in containers that have the same volume V and are kept at the same temperature T, approximately all have the same pressure p. The small differences in pressure disappear if lower gas densities are used. Further experiments showed that all low-density gases obey the equation pV = nRT. Here R = 8.31 K/mol ⋅ K and is known as the "gas constant." The equation itself is known as the "ideal gas law." The constant R can be expressed -23 as R = kNA . Here k is called the Boltzmann constant and is equal to 1.38 × 10 J/K. N If we substitute R as well as n = in the ideal gas law we get the equivalent form: NA pV = NkT. Here N is the number of molecules in the gas. The behavior of all real gases approaches that of an ideal gas at low enough densities. Low densitiens m= enumberans tha oft t hemoles gas molecul es are fa Nr e=nough number apa ofr tparticles that the y do not interact with one another, but only with the walls of the gas container. -

Philosophical Magazine Vol
Philosophical Magazine Vol. 92, Nos. 1–3, 1–21 January 2012, 353–361 Exploring models of associative memory via cavity quantum electrodynamics Sarang Gopalakrishnana, Benjamin L. Levb and Paul M. Goldbartc* aDepartment of Physics, University of Illinois at Urbana-Champaign, 1110 West Green Street Urbana, Illinois 61801, USA; bDepartments of Applied Physics and Physics and E. L. Ginzton Laboratory, Stanford University, Stanford, CA 94305, USA; cSchool of Physics, Georgia Institute of Technology, 837 State Street, Atlanta, GA 30332, USA (Received 2 August 2011; final version received 24 October 2011) Photons in multimode optical cavities can be used to mediate tailored interactions between atoms confined in the cavities. For atoms possessing multiple internal (i.e., ‘‘spin’’) states, the spin–spin interactions mediated by the cavity are analogous in structure to the Ruderman–Kittel–Kasuya– Yosida (RKKY) interaction between localized spins in metals. Thus, in particular, it is possible to use atoms in cavities to realize models of frustrated and/or disordered spin systems, including models that can be mapped on to the Hopfield network model and related models of associative memory. We explain how this realization of models of associative memory comes about and discuss ways in which the properties of these models can be probed in a cavity-based setting. Keywords: disordered systems; magnetism; statistical physics; ultracold atoms; neural networks; spin glasses 1. Introductory remarks Since the development of laser cooling and trapping, a -

IB Questionbank
Topic 3 Past Paper [94 marks] This question is about thermal energy transfer. A hot piece of iron is placed into a container of cold water. After a time the iron and water reach thermal equilibrium. The heat capacity of the container is negligible. specific heat capacity. [2 marks] 1a. Define Markscheme the energy required to change the temperature (of a substance) by 1K/°C/unit degree; of mass 1 kg / per unit mass; [5 marks] 1b. The following data are available. Mass of water = 0.35 kg Mass of iron = 0.58 kg Specific heat capacity of water = 4200 J kg–1K–1 Initial temperature of water = 20°C Final temperature of water = 44°C Initial temperature of iron = 180°C (i) Determine the specific heat capacity of iron. (ii) Explain why the value calculated in (b)(i) is likely to be different from the accepted value. Markscheme (i) use of mcΔT; 0.58×c×[180-44]=0.35×4200×[44-20]; c=447Jkg-1K-1≈450Jkg-1K-1; (ii) energy would be given off to surroundings/environment / energy would be absorbed by container / energy would be given off through vaporization of water; hence final temperature would be less; hence measured value of (specific) heat capacity (of iron) would be higher; This question is in two parts. Part 1 is about ideal gases and specific heat capacity. Part 2 is about simple harmonic motion and waves. Part 1 Ideal gases and specific heat capacity State assumptions of the kinetic model of an ideal gas. [2 marks] 2a. two Markscheme point molecules / negligible volume; no forces between molecules except during contact; motion/distribution is random; elastic collisions / no energy lost; obey Newton’s laws of motion; collision in zero time; gravity is ignored; [4 marks] 2b. -

Thermodynamics of Ideal Gases
D Thermodynamics of ideal gases An ideal gas is a nice “laboratory” for understanding the thermodynamics of a fluid with a non-trivial equation of state. In this section we shall recapitulate the conventional thermodynamics of an ideal gas with constant heat capacity. For more extensive treatments, see for example [67, 66]. D.1 Internal energy In section 4.1 we analyzed Bernoulli’s model of a gas consisting of essentially 1 2 non-interacting point-like molecules, and found the pressure p = 3 ½ v where v is the root-mean-square average molecular speed. Using the ideal gas law (4-26) the total molecular kinetic energy contained in an amount M = ½V of the gas becomes, 1 3 3 Mv2 = pV = nRT ; (D-1) 2 2 2 where n = M=Mmol is the number of moles in the gas. The derivation in section 4.1 shows that the factor 3 stems from the three independent translational degrees of freedom available to point-like molecules. The above formula thus expresses 1 that in a mole of a gas there is an internal kinetic energy 2 RT associated with each translational degree of freedom of the point-like molecules. Whereas monatomic gases like argon have spherical molecules and thus only the three translational degrees of freedom, diatomic gases like nitrogen and oxy- Copyright °c 1998{2004, Benny Lautrup Revision 7.7, January 22, 2004 662 D. THERMODYNAMICS OF IDEAL GASES gen have stick-like molecules with two extra rotational degrees of freedom or- thogonally to the bridge connecting the atoms, and multiatomic gases like carbon dioxide and methane have the three extra rotational degrees of freedom. -
![Arxiv:0801.0028V1 [Physics.Atom-Ph] 29 Dec 2007 § ‡ † (People’S China Metrology, Of) of Lic Institute Canada National Council, Zhang, Research Z](https://docslib.b-cdn.net/cover/1910/arxiv-0801-0028v1-physics-atom-ph-29-dec-2007-%C2%A7-people-s-china-metrology-of-of-lic-institute-canada-national-council-zhang-research-z-651910.webp)
Arxiv:0801.0028V1 [Physics.Atom-Ph] 29 Dec 2007 § ‡ † (People’S China Metrology, Of) of Lic Institute Canada National Council, Zhang, Research Z
CODATA Recommended Values of the Fundamental Physical Constants: 2006∗ Peter J. Mohr†, Barry N. Taylor‡, and David B. Newell§, National Institute of Standards and Technology, Gaithersburg, Maryland 20899-8420, USA (Dated: March 29, 2012) This paper gives the 2006 self-consistent set of values of the basic constants and conversion factors of physics and chemistry recommended by the Committee on Data for Science and Technology (CODATA) for international use. Further, it describes in detail the adjustment of the values of the constants, including the selection of the final set of input data based on the results of least-squares analyses. The 2006 adjustment takes into account the data considered in the 2002 adjustment as well as the data that became available between 31 December 2002, the closing date of that adjustment, and 31 December 2006, the closing date of the new adjustment. The new data have led to a significant reduction in the uncertainties of many recommended values. The 2006 set replaces the previously recommended 2002 CODATA set and may also be found on the World Wide Web at physics.nist.gov/constants. Contents 3. Cyclotron resonance measurement of the electron relative atomic mass Ar(e) 8 Glossary 2 4. Atomic transition frequencies 8 1. Introduction 4 1. Hydrogen and deuterium transition frequencies, the 1. Background 4 Rydberg constant R∞, and the proton and deuteron charge radii R , R 8 2. Time variation of the constants 5 p d 1. Theory relevant to the Rydberg constant 9 3. Outline of paper 5 2. Experiments on hydrogen and deuterium 16 3. -

Conventioral Standards?* JESSE W
156 IRE TRANSACTIONS ON INSTRUMENTATION December Present Status of Precise Information on the Universal Physical Constants. Has the Time Arrived for Their Adoption to Replace Our Present Arbitrary Conventioral Standards?* JESSE W. M. DuMONDt INTRODUCTION this subject that the experimentally measured data r HREE years ago Dr. E. R. Cohen and I prepared often do not give the desired unknowns directly but and published our latest (1955) least-squares ad- instead give functions of the unknowns. justment of all the most reliable data then avail- Seven different functions of these above four un- able bearing on the universal constants of physics and knowns have been measured by experimental methods chemistry. Since then new data and information have which we feel are sufficiently precise and reliable to been accumulating so that a year or two from now the qualify them as input data in a least-squares adjust- time may perhaps be propitious for us to prepare a new ment. These seven experimentally determined numeri- adjustment taking the newly-gained knowledge into cal values are not only functions of the unknowns,ae, account. At present it is too early to attempt such a e, N, and A, but also of the above-mentioned experi re-evaluation since many of the investigations and re- mentally determined auxiliary constants, of which five determinations now under way are still far from com- different kinds are listed in Table I. Another of these pleted. I shall be obliged, therefore, to content myself auxiliary constants I find it expedient to recall to your in this talk with a description of the sources of informa- attention at the very beginning to avoid any possibility tion upon which our 1955 evaluation was based, men- of confusion. -

5.5 ENTROPY CHANGES of an IDEAL GAS for One Mole Or a Unit Mass of Fluid Undergoing a Mechanically Reversible Process in a Closed System, the First Law, Eq
Thermodynamic Third class Dr. Arkan J. Hadi 5.5 ENTROPY CHANGES OF AN IDEAL GAS For one mole or a unit mass of fluid undergoing a mechanically reversible process in a closed system, the first law, Eq. (2.8), becomes: Differentiation of the defining equation for enthalpy, H = U + PV, yields: Eliminating dU gives: For an ideal gas, dH = and V = RT/ P. With these substitutions and then division by T, As a result of Eq. (5.11), this becomes: where S is the molar entropy of an ideal gas. Integration from an initial state at conditions To and Po to a final state at conditions T and P gives: Although derived for a mechanically reversible process, this equation relates properties only, and is independent of the process causing the change of state. It is therefore a general equation for the calculation of entropy changes of an ideal gas. 1 Thermodynamic Third class Dr. Arkan J. Hadi 5.6 MATHEMATICAL STATEMENT OF THE SECOND LAW Consider two heat reservoirs, one at temperature TH and a second at the lower temperature Tc. Let a quantity of heat | | be transferred from the hotter to the cooler reservoir. The entropy changes of the reservoirs at TH and at Tc are: These two entropy changes are added to give: Since TH > Tc, the total entropy change as a result of this irreversible process is positive. Also, ΔStotal becomes smaller as the difference TH - TC gets smaller. When TH is only infinitesimally higher than Tc, the heat transfer is reversible, and ΔStotal approaches zero. Thus for the process of irreversible heat transfer, ΔStotal is always positive, approaching zero as the process becomes reversible. -

Chapter 3 3.4-2 the Compressibility Factor Equation of State
Chapter 3 3.4-2 The Compressibility Factor Equation of State The dimensionless compressibility factor, Z, for a gaseous species is defined as the ratio pv Z = (3.4-1) RT If the gas behaves ideally Z = 1. The extent to which Z differs from 1 is a measure of the extent to which the gas is behaving nonideally. The compressibility can be determined from experimental data where Z is plotted versus a dimensionless reduced pressure pR and reduced temperature TR, defined as pR = p/pc and TR = T/Tc In these expressions, pc and Tc denote the critical pressure and temperature, respectively. A generalized compressibility chart of the form Z = f(pR, TR) is shown in Figure 3.4-1 for 10 different gases. The solid lines represent the best curves fitted to the data. Figure 3.4-1 Generalized compressibility chart for various gases10. It can be seen from Figure 3.4-1 that the value of Z tends to unity for all temperatures as pressure approach zero and Z also approaches unity for all pressure at very high temperature. If the p, v, and T data are available in table format or computer software then you should not use the generalized compressibility chart to evaluate p, v, and T since using Z is just another approximation to the real data. 10 Moran, M. J. and Shapiro H. N., Fundamentals of Engineering Thermodynamics, Wiley, 2008, pg. 112 3-19 Example 3.4-2 ---------------------------------------------------------------------------------- A closed, rigid tank filled with water vapor, initially at 20 MPa, 520oC, is cooled until its temperature reaches 400oC. -

Philosophical Magazine Atomic-Resolution Spectroscopic
This article was downloaded by: [Cornell University Library] On: 30 April 2015, At: 08:23 Publisher: Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK Philosophical Magazine Publication details, including instructions for authors and subscription information: http://www.tandfonline.com/loi/tphm20 Atomic-resolution spectroscopic imaging of oxide interfaces L. Fitting Kourkoutis a , H.L. Xin b , T. Higuchi c , Y. Hotta c d , J.H. Lee e f , Y. Hikita c , D.G. Schlom e , H.Y. Hwang c g & D.A. Muller a h a School of Applied and Engineering Physics , Cornell University , Ithaca, USA b Department of Physics , Cornell University , Ithaca, USA c Department of Advanced Materials Science , University of Tokyo , Kashiwa, Japan d Correlated Electron Research Group , RIKEN , Saitama, Japan e Department of Materials Science and Engineering , Cornell University , Ithaca, USA f Department of Materials Science and Engineering , Pennsylvania State University , University Park, USA g Japan Science and Technology Agency , Kawaguchi, Japan h Kavli Institute at Cornell for Nanoscale Science , Ithaca, USA Published online: 20 Oct 2010. To cite this article: L. Fitting Kourkoutis , H.L. Xin , T. Higuchi , Y. Hotta , J.H. Lee , Y. Hikita , D.G. Schlom , H.Y. Hwang & D.A. Muller (2010) Atomic-resolution spectroscopic imaging of oxide interfaces, Philosophical Magazine, 90:35-36, 4731-4749, DOI: 10.1080/14786435.2010.518983 To link to this article: http://dx.doi.org/10.1080/14786435.2010.518983 PLEASE SCROLL DOWN FOR ARTICLE Taylor & Francis makes every effort to ensure the accuracy of all the information (the “Content”) contained in the publications on our platform. -

Module P7.4 Specific Heat, Latent Heat and Entropy
FLEXIBLE LEARNING APPROACH TO PHYSICS Module P7.4 Specific heat, latent heat and entropy 1 Opening items 4 PVT-surfaces and changes of phase 1.1 Module introduction 4.1 The critical point 1.2 Fast track questions 4.2 The triple point 1.3 Ready to study? 4.3 The Clausius–Clapeyron equation 2 Heating solids and liquids 5 Entropy and the second law of thermodynamics 2.1 Heat, work and internal energy 5.1 The second law of thermodynamics 2.2 Changes of temperature: specific heat 5.2 Entropy: a function of state 2.3 Changes of phase: latent heat 5.3 The principle of entropy increase 2.4 Measuring specific heats and latent heats 5.4 The irreversibility of nature 3 Heating gases 6 Closing items 3.1 Ideal gases 6.1 Module summary 3.2 Principal specific heats: monatomic ideal gases 6.2 Achievements 3.3 Principal specific heats: other gases 6.3 Exit test 3.4 Isothermal and adiabatic processes Exit module FLAP P7.4 Specific heat, latent heat and entropy COPYRIGHT © 1998 THE OPEN UNIVERSITY S570 V1.1 1 Opening items 1.1 Module introduction What happens when a substance is heated? Its temperature may rise; it may melt or evaporate; it may expand and do work1—1the net effect of the heating depends on the conditions under which the heating takes place. In this module we discuss the heating of solids, liquids and gases under a variety of conditions. We also look more generally at the problem of converting heat into useful work, and the related issue of the irreversibility of many natural processes. -

Real Gases – As Opposed to a Perfect Or Ideal Gas – Exhibit Properties That Cannot Be Explained Entirely Using the Ideal Gas Law
Basic principle II Second class Dr. Arkan Jasim Hadi 1. Real gas Real gases – as opposed to a perfect or ideal gas – exhibit properties that cannot be explained entirely using the ideal gas law. To understand the behavior of real gases, the following must be taken into account: compressibility effects; variable specific heat capacity; van der Waals forces; non-equilibrium thermodynamic effects; Issues with molecular dissociation and elementary reactions with variable composition. Critical state and Reduced conditions Critical point: The point at highest temp. (Tc) and Pressure (Pc) at which a pure chemical species can exist in vapour/liquid equilibrium. The point critical is the point at which the liquid and vapour phases are not distinguishable; because of the liquid and vapour having same properties. Reduced properties of a fluid are a set of state variables normalized by the fluid's state properties at its critical point. These dimensionless thermodynamic coordinates, taken together with a substance's compressibility factor, provide the basis for the simplest form of the theorem of corresponding states The reduced pressure is defined as its actual pressure divided by its critical pressure : The reduced temperature of a fluid is its actual temperature, divided by its critical temperature: The reduced specific volume ") of a fluid is computed from the ideal gas law at the substance's critical pressure and temperature: This property is useful when the specific volume and either temperature or pressure are known, in which case the missing third property can be computed directly. 1 Basic principle II Second class Dr. Arkan Jasim Hadi In Kay's method, pseudocritical values for mixtures of gases are calculated on the assumption that each component in the mixture contributes to the pseudocritical value in the same proportion as the mol fraction of that component in the gas. -
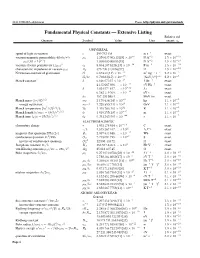
Fundamental Physical Constants — Extensive Listing Relative Std
2018 CODATA adjustment From: http://physics.nist.gov/constants Fundamental Physical Constants — Extensive Listing Relative std. Quantity Symbol Value Unit uncert. ur UNIVERSAL speed of light in vacuum c 299 792 458 m s−1 exact 2 −6 −2 −10 vacuum magnetic permeability 4pα¯h=e c µ0 1:256 637 062 12(19) × 10 NA 1:5 × 10 −7 −2 −10 µ0=(4p × 10 ) 1:000 000 000 55(15) NA 1:5 × 10 2 −12 −1 −10 vacuum electric permittivity 1/µ0c 0 8:854 187 8128(13) × 10 F m 1:5 × 10 −10 characteristic impedance of vacuum µ0c Z0 376:730 313 668(57) Ω 1:5 × 10 Newtonian constant of gravitation G 6:674 30(15) × 10−11 m3 kg−1 s−2 2:2 × 10−5 G=¯hc 6:708 83(15) × 10−39 (GeV=c2)−2 2:2 × 10−5 Planck constant∗ h 6:626 070 15 × 10−34 J Hz−1 exact 4:135 667 696 ::: × 10−15 eV Hz−1 exact ¯h 1:054 571 817 ::: × 10−34 J s exact 6:582 119 569 ::: × 10−16 eV s exact ¯hc 197:326 980 4 ::: MeV fm exact 1=2 −8 −5 Planck mass (¯hc=G) mP 2:176 434(24) × 10 kg 1:1 × 10 2 19 −5 energy equivalent mPc 1:220 890(14) × 10 GeV 1:1 × 10 5 1=2 32 −5 Planck temperature (¯hc =G) =k TP 1:416 784(16) × 10 K 1:1 × 10 3 1=2 −35 −5 Planck length ¯h=mPc = (¯hG=c ) lP 1:616 255(18) × 10 m 1:1 × 10 5 1=2 −44 −5 Planck time lP=c = (¯hG=c ) tP 5:391 247(60) × 10 s 1:1 × 10 ELECTROMAGNETIC elementary charge e 1:602 176 634 × 10−19 C exact e=¯h 1:519 267 447 ::: × 1015 AJ−1 exact −15 magnetic flux quantum 2p¯h=(2e) Φ0 2:067 833 848 ::: × 10 Wb exact 2 −5 conductance quantum 2e =2p¯h G0 7:748 091 729 ::: × 10 S exact −1 inverse of conductance quantum G0 12 906:403 72 ::: Ω exact 9 −1 Josephson constant 2e=h