Fundamental Constants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Arxiv:0801.0028V1 [Physics.Atom-Ph] 29 Dec 2007 § ‡ † (People’S China Metrology, Of) of Lic Institute Canada National Council, Zhang, Research Z](https://docslib.b-cdn.net/cover/1910/arxiv-0801-0028v1-physics-atom-ph-29-dec-2007-%C2%A7-people-s-china-metrology-of-of-lic-institute-canada-national-council-zhang-research-z-651910.webp)
Arxiv:0801.0028V1 [Physics.Atom-Ph] 29 Dec 2007 § ‡ † (People’S China Metrology, Of) of Lic Institute Canada National Council, Zhang, Research Z
CODATA Recommended Values of the Fundamental Physical Constants: 2006∗ Peter J. Mohr†, Barry N. Taylor‡, and David B. Newell§, National Institute of Standards and Technology, Gaithersburg, Maryland 20899-8420, USA (Dated: March 29, 2012) This paper gives the 2006 self-consistent set of values of the basic constants and conversion factors of physics and chemistry recommended by the Committee on Data for Science and Technology (CODATA) for international use. Further, it describes in detail the adjustment of the values of the constants, including the selection of the final set of input data based on the results of least-squares analyses. The 2006 adjustment takes into account the data considered in the 2002 adjustment as well as the data that became available between 31 December 2002, the closing date of that adjustment, and 31 December 2006, the closing date of the new adjustment. The new data have led to a significant reduction in the uncertainties of many recommended values. The 2006 set replaces the previously recommended 2002 CODATA set and may also be found on the World Wide Web at physics.nist.gov/constants. Contents 3. Cyclotron resonance measurement of the electron relative atomic mass Ar(e) 8 Glossary 2 4. Atomic transition frequencies 8 1. Introduction 4 1. Hydrogen and deuterium transition frequencies, the 1. Background 4 Rydberg constant R∞, and the proton and deuteron charge radii R , R 8 2. Time variation of the constants 5 p d 1. Theory relevant to the Rydberg constant 9 3. Outline of paper 5 2. Experiments on hydrogen and deuterium 16 3. -

Conventioral Standards?* JESSE W
156 IRE TRANSACTIONS ON INSTRUMENTATION December Present Status of Precise Information on the Universal Physical Constants. Has the Time Arrived for Their Adoption to Replace Our Present Arbitrary Conventioral Standards?* JESSE W. M. DuMONDt INTRODUCTION this subject that the experimentally measured data r HREE years ago Dr. E. R. Cohen and I prepared often do not give the desired unknowns directly but and published our latest (1955) least-squares ad- instead give functions of the unknowns. justment of all the most reliable data then avail- Seven different functions of these above four un- able bearing on the universal constants of physics and knowns have been measured by experimental methods chemistry. Since then new data and information have which we feel are sufficiently precise and reliable to been accumulating so that a year or two from now the qualify them as input data in a least-squares adjust- time may perhaps be propitious for us to prepare a new ment. These seven experimentally determined numeri- adjustment taking the newly-gained knowledge into cal values are not only functions of the unknowns,ae, account. At present it is too early to attempt such a e, N, and A, but also of the above-mentioned experi re-evaluation since many of the investigations and re- mentally determined auxiliary constants, of which five determinations now under way are still far from com- different kinds are listed in Table I. Another of these pleted. I shall be obliged, therefore, to content myself auxiliary constants I find it expedient to recall to your in this talk with a description of the sources of informa- attention at the very beginning to avoid any possibility tion upon which our 1955 evaluation was based, men- of confusion. -

The Discovery of Thermodynamics
Philosophical Magazine ISSN: 1478-6435 (Print) 1478-6443 (Online) Journal homepage: https://www.tandfonline.com/loi/tphm20 The discovery of thermodynamics Peter Weinberger To cite this article: Peter Weinberger (2013) The discovery of thermodynamics, Philosophical Magazine, 93:20, 2576-2612, DOI: 10.1080/14786435.2013.784402 To link to this article: https://doi.org/10.1080/14786435.2013.784402 Published online: 09 Apr 2013. Submit your article to this journal Article views: 658 Citing articles: 2 View citing articles Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=tphm20 Philosophical Magazine, 2013 Vol. 93, No. 20, 2576–2612, http://dx.doi.org/10.1080/14786435.2013.784402 COMMENTARY The discovery of thermodynamics Peter Weinberger∗ Center for Computational Nanoscience, Seilerstätte 10/21, A1010 Vienna, Austria (Received 21 December 2012; final version received 6 March 2013) Based on the idea that a scientific journal is also an “agora” (Greek: market place) for the exchange of ideas and scientific concepts, the history of thermodynamics between 1800 and 1910 as documented in the Philosophical Magazine Archives is uncovered. Famous scientists such as Joule, Thomson (Lord Kelvin), Clau- sius, Maxwell or Boltzmann shared this forum. Not always in the most friendly manner. It is interesting to find out, how difficult it was to describe in a scientific (mathematical) language a phenomenon like “heat”, to see, how long it took to arrive at one of the fundamental principles in physics: entropy. Scientific progress started from the simple rule of Boyle and Mariotte dating from the late eighteenth century and arrived in the twentieth century with the concept of probabilities. -
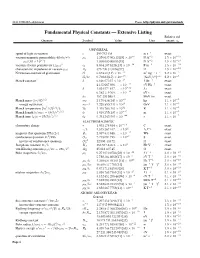
Fundamental Physical Constants — Extensive Listing Relative Std
2018 CODATA adjustment From: http://physics.nist.gov/constants Fundamental Physical Constants — Extensive Listing Relative std. Quantity Symbol Value Unit uncert. ur UNIVERSAL speed of light in vacuum c 299 792 458 m s−1 exact 2 −6 −2 −10 vacuum magnetic permeability 4pα¯h=e c µ0 1:256 637 062 12(19) × 10 NA 1:5 × 10 −7 −2 −10 µ0=(4p × 10 ) 1:000 000 000 55(15) NA 1:5 × 10 2 −12 −1 −10 vacuum electric permittivity 1/µ0c 0 8:854 187 8128(13) × 10 F m 1:5 × 10 −10 characteristic impedance of vacuum µ0c Z0 376:730 313 668(57) Ω 1:5 × 10 Newtonian constant of gravitation G 6:674 30(15) × 10−11 m3 kg−1 s−2 2:2 × 10−5 G=¯hc 6:708 83(15) × 10−39 (GeV=c2)−2 2:2 × 10−5 Planck constant∗ h 6:626 070 15 × 10−34 J Hz−1 exact 4:135 667 696 ::: × 10−15 eV Hz−1 exact ¯h 1:054 571 817 ::: × 10−34 J s exact 6:582 119 569 ::: × 10−16 eV s exact ¯hc 197:326 980 4 ::: MeV fm exact 1=2 −8 −5 Planck mass (¯hc=G) mP 2:176 434(24) × 10 kg 1:1 × 10 2 19 −5 energy equivalent mPc 1:220 890(14) × 10 GeV 1:1 × 10 5 1=2 32 −5 Planck temperature (¯hc =G) =k TP 1:416 784(16) × 10 K 1:1 × 10 3 1=2 −35 −5 Planck length ¯h=mPc = (¯hG=c ) lP 1:616 255(18) × 10 m 1:1 × 10 5 1=2 −44 −5 Planck time lP=c = (¯hG=c ) tP 5:391 247(60) × 10 s 1:1 × 10 ELECTROMAGNETIC elementary charge e 1:602 176 634 × 10−19 C exact e=¯h 1:519 267 447 ::: × 1015 AJ−1 exact −15 magnetic flux quantum 2p¯h=(2e) Φ0 2:067 833 848 ::: × 10 Wb exact 2 −5 conductance quantum 2e =2p¯h G0 7:748 091 729 ::: × 10 S exact −1 inverse of conductance quantum G0 12 906:403 72 ::: Ω exact 9 −1 Josephson constant 2e=h -

Fundamental Physical Constants — Complete Listing Relative Std
From: http://physics.nist.gov/constants Fundamental Physical Constants — Complete Listing Relative std. Quantity Symbol Value Unit uncert. ur UNIVERSAL 1 speed of light in vacuum c; c0 299 792 458 m s− (exact) 7 2 magnetic constant µ0 4π 10− NA− × 7 2 = 12:566 370 614::: 10− NA− (exact) 2 × 12 1 electric constant 1/µ0c "0 8:854 187 817::: 10− F m− (exact) characteristic impedance × of vacuum µ / = µ c Z 376:730 313 461::: Ω (exact) p 0 0 0 0 Newtonian constant 11 3 1 2 3 of gravitation G 6:673(10) 10− m kg− s− 1:5 10− × 39 2 2 × 3 G=¯hc 6:707(10) 10− (GeV=c )− 1:5 10− × 34 × 8 Planck constant h 6:626 068 76(52) 10− J s 7:8 10− × 15 × 8 in eV s 4:135 667 27(16) 10− eV s 3:9 10− × 34 × 8 h=2π ¯h 1:054 571 596(82) 10− J s 7:8 10− × 16 × 8 in eV s 6:582 118 89(26) 10− eV s 3:9 10− × × 1=2 8 4 Planck mass (¯hc=G) mP 2:1767(16) 10− kg 7:5 10− 3 1=2 × 35 × 4 Planck length ¯h=mPc = (¯hG=c ) lP 1:6160(12) 10− m 7:5 10− 5 1=2 × 44 × 4 Planck time lP=c = (¯hG=c ) tP 5:3906(40) 10− s 7:5 10− × × ELECTROMAGNETIC 19 8 elementary charge e 1:602 176 462(63) 10− C 3:9 10− × 14 1 × 8 e=h 2:417 989 491(95) 10 AJ− 3:9 10− × × 15 8 magnetic flux quantum h=2e Φ0 2:067 833 636(81) 10− Wb 3:9 10− 2 × 5 × 9 conductance quantum 2e =h G0 7:748 091 696(28) 10− S 3:7 10− 1 × × 9 inverse of conductance quantum G0− 12 906:403 786(47) Ω 3:7 10− a 9 1 × 8 Josephson constant 2e=h KJ 483 597:898(19) 10 Hz V− 3:9 10− von Klitzing constantb × × 2 9 h=e = µ0c=2α RK 25 812:807 572(95) Ω 3:7 10− × 26 1 8 Bohr magneton e¯h=2me µB 927:400 899(37) 10− JT− 4:0 10− 1 × 5 1 × 9 in -

List of Symbols
Appendix A List of Symbols For convenience, many symbols frequently used in the text are listed below alphabetically. Some of the symbols may play a dual role, in which case the meaning should be obvious from the context. A number in parentheses refers to an Eq. number (or the text or footnote near that Eq. number) in which the symbol is defined or appears for the first time; in a few cases a page number, Table number, Sect. number, footnote (fn.) number, or Fig. number is given instead. Quantum number is abbreviated as q. n. Quantum numbers (atomic): Symbol Description Eq. No. j D l ˙ s Inner q. n. of an electron (3.13) J D L C S Inner (total angular momentum) q. n. of a state (5.51b) l P Azimuthal (orbital) q. n. of an electron (3.1) and (3.2) L D l Azimuthal (orbital) q. n. of a state (5.51c) ml Magnetic orbital q. n. of an electron (3.1) ms Magnetic spin q. n. of an electron (3.1) M Total magnetic q. n. (5.51d) n Principal q. n. of an electron (3.1) s P Spin q. n. of an electron (3.13) S D s Spin q. n. of a state (5.51e) Relativistic azimuthal q. n. of an electron (3.15a) and (3.15b) Relativistic magnetic q. n. of an electron (3.18a) and (3.18b) W.F. Huebner and W.D. Barfield, Opacity, Astrophysics and Space Science Library 402, 457 DOI 10.1007/978-1-4614-8797-5, © Springer Science+Business Media New York 2014 458 Appendix A: List of Symbols Quantum numbers (molecular)1: Symbol Description Eq. -

Chapter 1 Elementary Kinetic Theory of Gases
Chapter 1 Elementary kinetic theory of gases 1.1. Introduction. Molecular description of gases 1.2. Molecular quantities and macroscopic gas parameters 1.3. Gas laws 1.4. Collision frequency. Free molecular, transitional, and continuum flow regimes 1.5. Transfer of molecular quantities 1.6. Transfer equation 1.7. Diffusion, viscous drag, and heat conduction 1.8. Appendix: Probability and Bernoulli trial ME 591, Non‐equilibrium gas dynamics, Alexey Volkov 1 1.1. Introduction. Molecular description of gases Subject of kinetic theory. What are we going to study? Historical perspective Hypothesis of molecular structure of gases Sizes of atoms and molecules Masses of atoms and molecules Properties of major components of atmospheric air and other gases Length and time scale of inter‐molecular collisions Characteristic distance between molecules in gases Density parameter. Dilute and dense gases Purpose of the kinetic theory Chaotic motion of molecules. Major approach of the kinetic theory Specific goals of the kinetic theory Applications of the kinetic theory and RGD ME 591, Non‐equilibrium gas dynamics, Alexey Volkov 2 1.1. Introduction. Molecular description of gases Subject of kinetic theory. What are we going to study? Kinetic theory of gases is a part of statistical physics where flows of gases are considered on the molecular level, i.e. on the level of individual molecules, and described in terms of changes of probabilities of various states of gas molecules in space and time based on known laws of interaction between individual molecules. Word “Kinetic” came from a Greek verb meaning "to move“ or “to induce a motion.” Rarefiedgasdynamics(RGD)is often used as a synonymous of the kinetic theory of gases. -

The First and a Recent Experimental Determination of Avogadro's Number
REVISTA ENLACE QUÍMICO, UNIVERSIDAD DE GUANAJUATO VOL 2 NO 8, NOVIEMBRE DEL 2009 No 04-2010-101813383300-102 ® The first and a recent experimental determination of Avogadro's number Luis Márquez-Jaime1, Guillaume Gondre1 and Sergio Márquez-Gamiño2*. 1 Department of Mechanics, The Royal Institute of Technology (Kungliga Tekniska Högskolan, KTH), SE-100 44 Stockholm, Sweden. [email protected] 2Departmento de Ciencias Aplicadas al Trabajo, Universidad de Guanajuato, A. Postal 1-607, León, Gto., 37000 México. [email protected] *Corresponding author When a friend as Jesús is gone, your feelings of loss are overwhelming! Such that yourself can be lost, until you realize he is still influencing your own decision-taking processes. He is here as a model to follow! Sergio. ABSTRACT It has been almost two centuries since Avogrado presented his hypothesis, then, more than fifty years later Austrian Loschmidt developed a method for measuring the diameter of air molecules which was a big step for calculating a first approximation of the amount of molecules or atoms per unit volume. Computing Avogadro’s with Loschmidt’s diameter results give ten times the real value. It was until the beginning of the 19th century when Jean Perrin measured an accurate value of Avogadro’s constant, which is the amount of elementary entities in one mole of substance. After him, many scientists have measured this constant using more accurate methods, one of them is the X ray diffraction method, which can reach accuracy of 99.99% using 28Si. Key words: Avogadro’s constant, Avogadro’s number, Loschmidt, Perrin, X-ray diffraction. -
![Arxiv:1904.07669V1 [Physics.Optics] 16 Apr 2019 Ihrptto Aeoeaini Iie Yvlepuls- Valve by Moreover, Limited Is Capability](https://docslib.b-cdn.net/cover/3961/arxiv-1904-07669v1-physics-optics-16-apr-2019-ihrptto-aeoeaini-iie-yvlepuls-valve-by-moreover-limited-is-capability-4983961.webp)
Arxiv:1904.07669V1 [Physics.Optics] 16 Apr 2019 Ihrptto Aeoeaini Iie Yvlepuls- Valve by Moreover, Limited Is Capability
Real-time monitoring via second-harmonic interferometry of a flow gas cell for laser wakefield acceleration F. Brandi,1,2, a) F. Giammanco,3,4 F. Conti,3,4 F. Sylla,5 G. Lambert,6 and L. A. Gizzi1 1)Intense Laser Irradiation Laboratory (ILIL), Istituto Nazionale di Ottica (INO-CNR), Via Moruzzi 1, 56124 Pisa, Italy 2)Istituto Italiano di Tecnologia (IIT), Via Morego 30, 16163 Genova, Italy 3)Dipartimento di Fisica, Universit`adegli Studi di Pisa, Largo B. Pontecorvo 3, 56127 Pisa, Italy 4)Plasma Diagnostics & Technologies Ltd., via Matteucci n.38/D, 56124 Pisa, Italy 5)SourceLAB SAS, 86 rue de Paris, 91400 Orsay, France 6)LOA, ENSTA ParisTech, CNRS, Ecole Polytechnique, Universit Paris-Saclay, 828 bd des Marchaux, 91762 Palaiseau Cedex, France (Dated: 17 April 2019) The use of a gas cell as a target for laser weakfield acceleration (LWFA) offers the possibility to obtain stable and manageable laser-plasma interaction process, a mandatory condition for practical applications of this emerging technique, especially in multi-stage accelerators. In order to obtain full control of the gas particle number density in the interaction region, thus allowing for a long term stable and manageable LWFA, real-time monitoring is necessary. In fact, the ideal gas law cannot be used to estimate the particle density inside the flow cell based on the preset backing pressure and the room temperature because the gas flow depends on several factors like tubing, regulators and valves in the gas supply system, as well as vacuum chamber volume and vacuum pump speed/throughput. Here, second-harmonic interferometry is applied to measure the particle number density inside a flow gas cell designed for LWFA. -

Quantities, Units and Symbols in Physical Chemistry Third Edition
INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY Physical and Biophysical Chemistry Division 1 7 2 ) + Quantities, Units and Symbols in Physical Chemistry Third Edition Prepared for publication by E. Richard Cohen Tomislav Cvitaš Jeremy G. Frey Bertil Holmström Kozo Kuchitsu Roberto Marquardt Ian Mills Franco Pavese Martin Quack Jürgen Stohner Herbert L. Strauss Michio Takami Anders J Thor The first and second editions were prepared for publication by Ian Mills Tomislav Cvitaš Klaus Homann Nikola Kallay Kozo Kuchitsu IUPAC 2007 Professor E. Richard Cohen Professor Tom Cvitaš 17735, Corinthian Drive University of Zagreb Encino, CA 91316-3704 Department of Chemistry USA Horvatovac 102a email: [email protected] HR-10000 Zagreb Croatia email: [email protected] Professor Jeremy G. Frey Professor Bertil Holmström University of Southampton Ulveliden 15 Department of Chemistry SE-41674 Göteborg Southampton, SO 17 1BJ Sweden United Kingdom email: [email protected] email: [email protected] Professor Kozo Kuchitsu Professor Roberto Marquardt Tokyo University of Agriculture and Technology Laboratoire de Chimie Quantique Graduate School of BASE Institut de Chimie Naka-cho, Koganei Université Louis Pasteur Tokyo 184-8588 4, Rue Blaise Pascal Japan F-67000 Strasbourg email: [email protected] France email: [email protected] Professor Ian Mills Professor Franco Pavese University of Reading Instituto Nazionale di Ricerca Metrologica (INRIM) Department of Chemistry strada delle Cacce 73-91 Reading, RG6 6AD I-10135 Torino United Kingdom Italia email: [email protected] email: [email protected] Professor Martin Quack Professor Jürgen Stohner ETH Zürich ZHAW Zürich University of Applied Sciences Physical Chemistry ICBC Institute of Chemistry & Biological Chemistry CH-8093 Zürich Campus Reidbach T, Einsiedlerstr. -

Thermodynamic Properties of Ideal Bose Gas Trapped in Different
Thermodynamic properties of ideal Bose gas trapped in different external power-law potentials under generalized uncertainty principle* Ya-Ting Wang, He-Ling Li† (School of Physics and Electronic-Electrical Engineering, Ningxia University, Yinchuan 750021, China) Abstract Significant evidence is available to support the quantum effects of gravity that leads to the generalized uncertainty principle (GUP) and the minimum observable length. Usually quantum mechanics, statistical physics doesn't take gravity into account. Thermodynamic properties of ideal Bose gases in different external power-law potentials are studied under the GUP with statistical physical method. Critical temperature, internal energy, heat capacity, entropy, particles number of ground state and excited state are calculated analytically to ideal Bose gases in the external potentials under the GUP. Below the critical temperature, taking the rubidium and sodium atoms ideal Bose gases whose particle densities are under standard and experimental conditions, respectively, as examples, the relations of internal energy, heat capacity and entropy with temperature are analyzed numerically. Theoretical and numerical calculations show that: 1) the GUP leads to an increase in the critical temperature. 2)When the temperature is lower than the critical temperature and slightly higher than 0K, the GUP's amendments to internal energy, heat capacity and entropy etc. are positive. As the temperature increases to a certain value, these amendments become negative. 3)The external potentials can increase or decrease the influence of the GUP on thermodynamic properties. When ε=1J, ε is the quantity that reflects the external potential intensity, and atomic density n = 2.687×1025m3, the GUP's amendments to the internal energy, heat capacity and entropy of the rubidium atoms ideal Bose gas first decrease and then increase with the increase of X (where X≡Σi1/ti is sum of the reciprocal of the exponents of the power function). -

CODATA Recommended Values of the Fundamental Physical Constants: 2014∗
CODATA Recommended Values of the Fundamental Physical Constants: 2014∗ Peter J. Mohr†, David B. Newell‡, Barry N. Taylor§ National Institute of Standards and Technology, Gaithersburg, Maryland 20899-8420, USA (Dated: 25 June 2015) This document gives the 2014 self-consistent set of values of the constants and conversion factors of physics and chemistry recommended by the Committee on Data for Science and Technology (CODATA). These values are based on a least-squares adjustment that takes into account all data available up to 31 December 2014. The recommended values may also be found at physics.nist.gov/constants. ∗This report was prepared by the authors under the auspices of the CODATA Task Group on Fundamental Constants. The members of the task group are: F. Cabiati, Istituto Nazionale di Ricerca Metrologica, Italy J. Fischer, Physikalisch-Technische Bundesanstalt, Germany J. Flowers (deceased), National Physical Laboratory, United King- dom K. Fujii, National Metrology Institute of Japan, Japan S. G. Karshenboim, Pulkovo Observatory, Russian Federation E. de Mirand´es, Bureau international des poids et mesures P. J. Mohr, National Institute of Standards and Technology, United States of America D. B. Newell, National Institute of Standards and Technology, United States of America F. Nez, Laboratoire Kastler-Brossel, France K. Pachucki, University of Warsaw, Poland T. J. Quinn, Bureau international des poids et mesures C. Thomas, Bureau international des poids et mesures B. N. Taylor, National Institute of Standards and Technology, United States of America B. M. Wood, National Research Council, Canada Z. Zhang, National Institute of Metrology, China (People’s Repub- lic of) †Electronic address: [email protected] ‡Electronic address: [email protected] §Electronic address: [email protected] 2 TABLE I An abbreviated list of the CODATA recommended values of the fundamental constants of physics and chemistry based on the 2014 adjustment.