CODATA Recommended Values of the Fundamental Physical Constants: 2014∗
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Doubly-Magic Storage Ring EDM Measurement Method
A doubly-magic storage ring EDM measurement method Richard Talman Cornell University, Ithaca, U.S.A. 10 December, 2018 arXiv:1812.05949v1 [physics.acc-ph] 14 Dec 2018 1 Abstract This paper discusses \doubly-magic trap" operation of storage rings with su- perimposed electric and magnetic bending, allowing spins in two beams to be frozen (at the same time, if necessary), and their application to electric dipole moment (EDM) measurement. Especially novel is the possibility of simultaneous storage in the same ring of frozen spin beams of two different particle types. A few doubly-magic cases have been found: One has an 86.62990502 MeV frozen spin proton beam and a 30.09255159 MeV frozen spin positron beam (with accu- racies matching their known magnetic moments) counter-circulating in the same storage ring. (Assuming the positron EDM to be negligibly small) the positron beam can be used to null the worst source of systematic EDM error|namely, the existence of unintentional and unknown average radial magnetic field < Br > which, acting on the MDM, causes spurious background spin precession indis- tinguishable from foreground EDM-induced precession. The resulting measured proton minus positron EDM difference is then independent of < Br >. This amounts to being a measurement of the proton EDM. Most doubly-magic features can be tested in one or more \small" EDM proto- type rings. One promising example is a doubly-magic proton-helion combination, which would measure the difference between helion (i.e. helium-3) and proton EDM's. This combination can be used in the near future for EDM measurement, for example in a 10 m bending radius ring, using only already well-understood and proven technology. -

2019 Redefinition of SI Base Units
2019 redefinition of SI base units A redefinition of SI base units is scheduled to come into force on 20 May 2019.[1][2] The kilogram, ampere, kelvin, and mole will then be defined by setting exact numerical values for the Planck constant (h), the elementary electric charge (e), the Boltzmann constant (k), and the Avogadro constant (NA), respectively. The metre and candela are already defined by physical constants, subject to correction to their present definitions. The new definitions aim to improve the SI without changing the size of any units, thus ensuring continuity with existing measurements.[3][4] In November 2018, the 26th General Conference on Weights and Measures (CGPM) unanimously approved these changes,[5][6] which the International Committee for Weights and Measures (CIPM) had proposed earlier that year.[7]:23 The previous major change of the metric system was in 1960 when the International System of Units (SI) was formally published. The SI is a coherent system structured around seven base units whose definitions are unconstrained by that of any other unit and another twenty-two named units derived from these base units. The metre was redefined in terms of the wavelength of a spectral line of a The SI system after the 2019 redefinition: krypton-86 radiation,[Note 1] making it derivable from universal natural Dependence of base unit definitions onphysical constants with fixed numerical values and on other phenomena, but the kilogram remained defined in terms of a physical prototype, base units. leaving it the only artefact upon which the SI unit definitions depend. The metric system was originally conceived as a system of measurement that was derivable from unchanging phenomena,[8] but practical limitations necessitated the use of artefacts (the prototype metre and prototype kilogram) when the metric system was first introduced in France in 1799. -
![Arxiv:0801.0028V1 [Physics.Atom-Ph] 29 Dec 2007 § ‡ † (People’S China Metrology, Of) of Lic Institute Canada National Council, Zhang, Research Z](https://docslib.b-cdn.net/cover/1910/arxiv-0801-0028v1-physics-atom-ph-29-dec-2007-%C2%A7-people-s-china-metrology-of-of-lic-institute-canada-national-council-zhang-research-z-651910.webp)
Arxiv:0801.0028V1 [Physics.Atom-Ph] 29 Dec 2007 § ‡ † (People’S China Metrology, Of) of Lic Institute Canada National Council, Zhang, Research Z
CODATA Recommended Values of the Fundamental Physical Constants: 2006∗ Peter J. Mohr†, Barry N. Taylor‡, and David B. Newell§, National Institute of Standards and Technology, Gaithersburg, Maryland 20899-8420, USA (Dated: March 29, 2012) This paper gives the 2006 self-consistent set of values of the basic constants and conversion factors of physics and chemistry recommended by the Committee on Data for Science and Technology (CODATA) for international use. Further, it describes in detail the adjustment of the values of the constants, including the selection of the final set of input data based on the results of least-squares analyses. The 2006 adjustment takes into account the data considered in the 2002 adjustment as well as the data that became available between 31 December 2002, the closing date of that adjustment, and 31 December 2006, the closing date of the new adjustment. The new data have led to a significant reduction in the uncertainties of many recommended values. The 2006 set replaces the previously recommended 2002 CODATA set and may also be found on the World Wide Web at physics.nist.gov/constants. Contents 3. Cyclotron resonance measurement of the electron relative atomic mass Ar(e) 8 Glossary 2 4. Atomic transition frequencies 8 1. Introduction 4 1. Hydrogen and deuterium transition frequencies, the 1. Background 4 Rydberg constant R∞, and the proton and deuteron charge radii R , R 8 2. Time variation of the constants 5 p d 1. Theory relevant to the Rydberg constant 9 3. Outline of paper 5 2. Experiments on hydrogen and deuterium 16 3. -

Conventioral Standards?* JESSE W
156 IRE TRANSACTIONS ON INSTRUMENTATION December Present Status of Precise Information on the Universal Physical Constants. Has the Time Arrived for Their Adoption to Replace Our Present Arbitrary Conventioral Standards?* JESSE W. M. DuMONDt INTRODUCTION this subject that the experimentally measured data r HREE years ago Dr. E. R. Cohen and I prepared often do not give the desired unknowns directly but and published our latest (1955) least-squares ad- instead give functions of the unknowns. justment of all the most reliable data then avail- Seven different functions of these above four un- able bearing on the universal constants of physics and knowns have been measured by experimental methods chemistry. Since then new data and information have which we feel are sufficiently precise and reliable to been accumulating so that a year or two from now the qualify them as input data in a least-squares adjust- time may perhaps be propitious for us to prepare a new ment. These seven experimentally determined numeri- adjustment taking the newly-gained knowledge into cal values are not only functions of the unknowns,ae, account. At present it is too early to attempt such a e, N, and A, but also of the above-mentioned experi re-evaluation since many of the investigations and re- mentally determined auxiliary constants, of which five determinations now under way are still far from com- different kinds are listed in Table I. Another of these pleted. I shall be obliged, therefore, to content myself auxiliary constants I find it expedient to recall to your in this talk with a description of the sources of informa- attention at the very beginning to avoid any possibility tion upon which our 1955 evaluation was based, men- of confusion. -

Forward Helion Scattering and Neutron Polarization N
Forward Helion Scattering and Neutron Polarization N. H. Buttimore Trinity College Dublin, Ireland Abstract. The elastic scattering of spin half helium-3 nuclei at small angles can show a sufficiently large analyzing power to enable the level of helion polarization to be evaluated. As the helion to a large extent inherits the polarization of its unpaired neutron the asymmetry observed in helion collisions can be transformed into a measurement of the polarization of its constituent neutron. Neutron polarimetry therefore relies upon understanding the spin dependence of the electromagnetic and hadronic interactions in the region of interference where there is an optimal analyzing power. Keywords: Neutron, spin polarization, helion, elastic scattering, asymmetry PACS: 11.80.Cr, 12.20.Ds, 13.40.Ks, 25.55.Ci, 13.85.Dz, 13.88.+e INTRODUCTION The spin polarized neutrons available from a polarized helium-3 beam would be very suitable for the study of polarized down quarks in various QCD processes particularly relating to transversity [1], nucleon spin structure [2], multi Pomeron exchange [3], gluon distributions [4], and additional dimensions [5]. Measuring the analyzing power in small angle elastic scattering of hadrons with spin provides an opportunity for evaluating the level of polarisation of incident helium-3 nuclei (helions) [6] thereby providing an effective neutron polarimeter [7]. Such a method relies upon an understanding of high energy spin dependence in diffractive processes [8]. Here, the interference of elastic hadronic and electromagnetic interactions in a suitable peripheral region enhances the size of the transverse spin asym- metry sufficiently to offer a tangible polarimeter for high energy helions. -

The Discovery of Thermodynamics
Philosophical Magazine ISSN: 1478-6435 (Print) 1478-6443 (Online) Journal homepage: https://www.tandfonline.com/loi/tphm20 The discovery of thermodynamics Peter Weinberger To cite this article: Peter Weinberger (2013) The discovery of thermodynamics, Philosophical Magazine, 93:20, 2576-2612, DOI: 10.1080/14786435.2013.784402 To link to this article: https://doi.org/10.1080/14786435.2013.784402 Published online: 09 Apr 2013. Submit your article to this journal Article views: 658 Citing articles: 2 View citing articles Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=tphm20 Philosophical Magazine, 2013 Vol. 93, No. 20, 2576–2612, http://dx.doi.org/10.1080/14786435.2013.784402 COMMENTARY The discovery of thermodynamics Peter Weinberger∗ Center for Computational Nanoscience, Seilerstätte 10/21, A1010 Vienna, Austria (Received 21 December 2012; final version received 6 March 2013) Based on the idea that a scientific journal is also an “agora” (Greek: market place) for the exchange of ideas and scientific concepts, the history of thermodynamics between 1800 and 1910 as documented in the Philosophical Magazine Archives is uncovered. Famous scientists such as Joule, Thomson (Lord Kelvin), Clau- sius, Maxwell or Boltzmann shared this forum. Not always in the most friendly manner. It is interesting to find out, how difficult it was to describe in a scientific (mathematical) language a phenomenon like “heat”, to see, how long it took to arrive at one of the fundamental principles in physics: entropy. Scientific progress started from the simple rule of Boyle and Mariotte dating from the late eighteenth century and arrived in the twentieth century with the concept of probabilities. -

Small-Scale Fusion Tackles Energy, Space Applications
NEWS FEATURE NEWS FEATURE Small-scalefusiontacklesenergy,spaceapplications Efforts are underway to exploit a strategy that could generate fusion with relative ease. M. Mitchell Waldrop, Science Writer On July 14, 2015, nine years and five billion kilometers Cohen explains, referring to the ionized plasma inside after liftoff, NASA’s New Horizons spacecraft passed the tube that’s emitting the flashes. So there are no the dwarf planet Pluto and its outsized moon Charon actual fusion reactions taking place; that’s not in his at almost 14 kilometers per second—roughly 20 times research plan until the mid-2020s, when he hopes to faster than a rifle bullet. be working with a more advanced prototype at least The images and data that New Horizons pains- three times larger than this one. takingly radioed back to Earth in the weeks that If that hope pans out and his future machine does followed revealed a pair of worlds that were far more indeed produce more greenhouse gas–free fusion en- varied and geologically active than anyone had ergy than it consumes, Cohen and his team will have thought possible. The revelations were breathtak- beaten the standard timetable for fusion by about a ing—and yet tinged with melancholy, because New decade—using a reactor that’s just a tiny fraction of Horizons was almost certain to be both the first and the size and cost of the huge, donut-shaped “tokamak” the last spacecraft to visit this fascinating world in devices that have long devoured most of the research our lifetimes. funding in this field. -
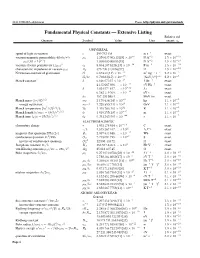
Fundamental Physical Constants — Extensive Listing Relative Std
2018 CODATA adjustment From: http://physics.nist.gov/constants Fundamental Physical Constants — Extensive Listing Relative std. Quantity Symbol Value Unit uncert. ur UNIVERSAL speed of light in vacuum c 299 792 458 m s−1 exact 2 −6 −2 −10 vacuum magnetic permeability 4pα¯h=e c µ0 1:256 637 062 12(19) × 10 NA 1:5 × 10 −7 −2 −10 µ0=(4p × 10 ) 1:000 000 000 55(15) NA 1:5 × 10 2 −12 −1 −10 vacuum electric permittivity 1/µ0c 0 8:854 187 8128(13) × 10 F m 1:5 × 10 −10 characteristic impedance of vacuum µ0c Z0 376:730 313 668(57) Ω 1:5 × 10 Newtonian constant of gravitation G 6:674 30(15) × 10−11 m3 kg−1 s−2 2:2 × 10−5 G=¯hc 6:708 83(15) × 10−39 (GeV=c2)−2 2:2 × 10−5 Planck constant∗ h 6:626 070 15 × 10−34 J Hz−1 exact 4:135 667 696 ::: × 10−15 eV Hz−1 exact ¯h 1:054 571 817 ::: × 10−34 J s exact 6:582 119 569 ::: × 10−16 eV s exact ¯hc 197:326 980 4 ::: MeV fm exact 1=2 −8 −5 Planck mass (¯hc=G) mP 2:176 434(24) × 10 kg 1:1 × 10 2 19 −5 energy equivalent mPc 1:220 890(14) × 10 GeV 1:1 × 10 5 1=2 32 −5 Planck temperature (¯hc =G) =k TP 1:416 784(16) × 10 K 1:1 × 10 3 1=2 −35 −5 Planck length ¯h=mPc = (¯hG=c ) lP 1:616 255(18) × 10 m 1:1 × 10 5 1=2 −44 −5 Planck time lP=c = (¯hG=c ) tP 5:391 247(60) × 10 s 1:1 × 10 ELECTROMAGNETIC elementary charge e 1:602 176 634 × 10−19 C exact e=¯h 1:519 267 447 ::: × 1015 AJ−1 exact −15 magnetic flux quantum 2p¯h=(2e) Φ0 2:067 833 848 ::: × 10 Wb exact 2 −5 conductance quantum 2e =2p¯h G0 7:748 091 729 ::: × 10 S exact −1 inverse of conductance quantum G0 12 906:403 72 ::: Ω exact 9 −1 Josephson constant 2e=h -

Fundamental Constants
Appendix A Fundamental Constants Table A.1 Fundamental constants Quantity & symbol CGS MKS (SI) Speed of light in vacuum c 2.997925 1010 cm sec−1 108 ms−1 −7 −6 −1 Magnetic constant μ0 = 4π × 10 1.25664 10 Hm (permeability of free space) 2 −12 −1 Electric constant 0 = 1/μ0c 8.8541853 10 Fm (permittivity of free space) Electron charge e 1.60219 10−19 C 4.80324 10−10 esu Electron volt eV 1.60219 10−12 erg eV−1 10−19 JeV−1 −28 −31 Electron rest mass m0 9.10956 10 gm 10 kg Planck’s constant h 6.626196 10−27 erg sec 10−34 Js Planck’s constant = h/2π 1.05459 10−27 erg sec 10−34 Js 2 2 −8 −10 Bohr radius a0 = 4π0 /m0e 0.529177 10 cm 10 m = 2 2 Rydberg constant Ry /2m0a0 13.6058 eV eV Fine structure constant 7.297351 10−3 10−3 2 α = e /20hc 23 −1 23 −1 Avogadro’s constant L,NA 6.022169 10 mol 10 mol 25 −3 Loschmidt’s constant NL 2.68719 10 m −16 −1 −23 −1 Boltzmann’s constant kB = R/L 1.380622 10 erg K 10 JK Faraday constant F = Le 9.64867 104 Cmol−1 7 −1 −1 −1 −1 Universal gas constant R = LkB 8.31435 10 erg K mol JK mol Mechanical equivalent of heat 4.184 107 erg cal−1 J cal−1 −21 −1 −24 −1 Bohr magneton μB = e/2m0c 9.2741 10 erg G 10 JT −24 −27 Proton rest mass mp 1.67251 10 gm 10 kg −24 −1 −27 −1 Nuclear magneton μN = e/2mpc 5.0508 10 erg G 10 JT −24 −27 Neutron rest mass mn 1.67492 10 gm 10 kg Stefan-Boltzmann constant 5.6697 10−8 Wm−2 K−4 = 5 4 3 2 σ 2π kB /15h c Y. -

Molar Mass 1 Molar Mass
Molar mass 1 Molar mass In chemistry, the molar mass is a physical property. It is defined as the mass of a given substance (chemical element or chemical compound) divided by its amount of substance. The base SI unit for molar mass is kg/mol. However, for historical reasons, molar masses are almost always expressed in g/mol. As an example, the molar mass of water is approximately: M(H O) ≈ 18 g⋅mol−1 2 Molar masses of elements The molar mass of atoms of an element is given by the atomic mass of the element multiplied by the molar mass constant, M −3 u = 1×10 kg/mol = 1 g/mol: M(H) = 1.007 97(7) × 1 g/mol = 1.007 97(7) g/mol M(S) = 32.065(5) × 1 g/mol = 32.065(5) g/mol M(Cl) = 35.453(2) × 1 g/mol = 35.453(2) g/mol M(Fe) = 55.845(2) × 1 g/mol = 55.845(2) g/mol. Multiplying by the molar mass constant ensures that the calculation is dimensionally correct: atomic weights are dimensionless quantities (i.e., pure numbers) whereas molar masses have units (in this case, grams/mole). Some elements are usually encountered as molecules, e.g. hydrogen (H 2), sulfur (S 8), chlorine (Cl 2). The molar mass of molecules of these elements is the molar mass of the atoms multiplied by the number of atoms in each molecule: M(H 2) = 2 × 1.007 97(7) × 1 g/mol = 2.015 88(14) g/mol M(S 8) = 8 × 32.065(5) × 1 g/mol = 256.52(4) g/mol M(Cl 2) = 2 × 35.453(2) × 1 g/mol = 70.906(4) g/mol. -

The Regulation of Fusion – a Practical and Innovation-Friendly Approach
The Regulation of Fusion – A Practical and Innovation-Friendly Approach February 2020 Amy C. Roma and Sachin S. Desai AUTHORS Amy C. Roma Sachin S. Desai Partner, Washington, D.C. Senior Associate, Washington, D.C. T +1 202 637 6831 T +1 202 637 3671 [email protected] [email protected] The authors want to sincerely thank the many stakeholders who provided feedback on this paper, and especially William Regan for his invaluable contributions and review of the technical discussion. TABLE OF CONTENTS I. EXECUTIVE SUMMARY 1 II. THE STATE OF FUSION INNOVATION 3 A) An Introduction to Fusion Energy 3 B) A Rapid Growth in Private-Sector Fusion Innovation 4 III. U.S. REGULATION OF ATOMIC ENERGY - NOT ONE SIZE FITS ALL 7 A) The Foundation of U.S. Nuclear Regulation - The Atomic Energy Act and the NRC 7 B) The Atomic Energy Act Embraces Different Regulations for Different Situations 7 1. NRC Frameworks for Different Safety Cases 8 2. Delegation of Regulatory Authority to States 9 IV. THE REGULATION OF FUSION - A PRACTICAL AND INNOVATION- FRIENDLY APPROACH 10 A) Fusion Regulation Comes to the Fore, Raising Key Questions 10 B) A Regulatory Proposal That Recognizes the Safety Case of Fusion and the Needs of Fusion Innovators 11 1. Near-Term: Regulation of Fusion Under the Part 30 Framework is Appropriate Through Development and Demonstration 11 2. Long-Term: The NRC Should Develop an Independent Regulatory Framework for Fusion at Commercial Scale, Not Adopt a Fission Framework 12 V. CONCLUSION 14 1 Hogan Lovells I. EXECUTIVE SUMMARY Fusion, the process that powers the Sun, has long been seen Most fusion technologies are already regulated by the NRC as the “holy grail” of energy production. -

Fundamental Physical Constants — Complete Listing Relative Std
From: http://physics.nist.gov/constants Fundamental Physical Constants — Complete Listing Relative std. Quantity Symbol Value Unit uncert. ur UNIVERSAL 1 speed of light in vacuum c; c0 299 792 458 m s− (exact) 7 2 magnetic constant µ0 4π 10− NA− × 7 2 = 12:566 370 614::: 10− NA− (exact) 2 × 12 1 electric constant 1/µ0c "0 8:854 187 817::: 10− F m− (exact) characteristic impedance × of vacuum µ / = µ c Z 376:730 313 461::: Ω (exact) p 0 0 0 0 Newtonian constant 11 3 1 2 3 of gravitation G 6:673(10) 10− m kg− s− 1:5 10− × 39 2 2 × 3 G=¯hc 6:707(10) 10− (GeV=c )− 1:5 10− × 34 × 8 Planck constant h 6:626 068 76(52) 10− J s 7:8 10− × 15 × 8 in eV s 4:135 667 27(16) 10− eV s 3:9 10− × 34 × 8 h=2π ¯h 1:054 571 596(82) 10− J s 7:8 10− × 16 × 8 in eV s 6:582 118 89(26) 10− eV s 3:9 10− × × 1=2 8 4 Planck mass (¯hc=G) mP 2:1767(16) 10− kg 7:5 10− 3 1=2 × 35 × 4 Planck length ¯h=mPc = (¯hG=c ) lP 1:6160(12) 10− m 7:5 10− 5 1=2 × 44 × 4 Planck time lP=c = (¯hG=c ) tP 5:3906(40) 10− s 7:5 10− × × ELECTROMAGNETIC 19 8 elementary charge e 1:602 176 462(63) 10− C 3:9 10− × 14 1 × 8 e=h 2:417 989 491(95) 10 AJ− 3:9 10− × × 15 8 magnetic flux quantum h=2e Φ0 2:067 833 636(81) 10− Wb 3:9 10− 2 × 5 × 9 conductance quantum 2e =h G0 7:748 091 696(28) 10− S 3:7 10− 1 × × 9 inverse of conductance quantum G0− 12 906:403 786(47) Ω 3:7 10− a 9 1 × 8 Josephson constant 2e=h KJ 483 597:898(19) 10 Hz V− 3:9 10− von Klitzing constantb × × 2 9 h=e = µ0c=2α RK 25 812:807 572(95) Ω 3:7 10− × 26 1 8 Bohr magneton e¯h=2me µB 927:400 899(37) 10− JT− 4:0 10− 1 × 5 1 × 9 in