Freezing Convenience Foods Include: • You Prepare Food When You Have Time
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Formula 1 Factsheet
Title: Herbalife Products Made Simple FactSheets 2013 ID: MA790 HL 2013 PMS-Fact Sheets-Core Nutrition_UK-EI (1 of 5) Proof No: B Date: 23/07/13 Nutritional Information Serving size: 26 g, Servings per container: 21 Per 100 ml of Per 250 ml of made up of made up HEALTHY Per 100 g product* product* % RDA** Energy 375 kcal 88 kcal (370 221 kcal - (1579 kJ) kJ) (925 kJ) Protein 34.6 g 7.0 g 17.4 g - Carbohydrate 34.6 g 8.6 g 21.4 g - MEAL of which: sugars 32.7 g 8.4 g 20.9 g - Fat 8.8 g 2.6 g 6.4 g - of which: saturated fatty acids 1.9 g 1.1 g 2.8 g - mono-unsaturated fatty acids 1.2 g 0.6 g 1.4 g - poly-unsaturated fatty acids 5.8 g 0.6 g 1.6 g - linoleic acid 3.9 g 0.4 g 1.1 g - Fibre 9.6 g 1.0 g 2.5 g - Sodium 0.52 g 0.10 g 0.26 g - VITAMINS Vitamin A 1211.54 µg 139.39 µg 348.48 µg 44 % Vitamin D 9.62 µg 1.00 µg 2.50 µg 50 % Vitamin E 19.23 mg 2.04 mg 5.10 mg 42 % Vitamin C 57.69 mg 7.75 mg 19.38 mg 24 % Thiamin 1.08 mg 0.15 mg 0.38 mg 34 % Riboflavin 1.62 mg 0.35 mg 0.88 mg 63 % EXCLUSIVELY AVAILABLE Niacin 20.77 mg 2.25 mg 5.63 mg 35 % THROUGH YOUR Vitamin B6 1.92 mg 0.25 mg 0.62 mg 44 % HERBALIFE Folic Acid 230.77 µg 28.64 µg 71.59 µg 36 % INDEPENDENT Vitamin B12 1.92 µg 0.63 µg 1.58 µg 63 % DISTRIBUTOR Biotin 96.15 µg 13.60 µg 34.01 µg 68 % Pantothenic Acid 6.54 mg 1.00 mg 2.50 mg 42 % MINERALS Calcium 384.62 mg 161.54 mg 403.85 mg 50 % Iron 20.00 mg 2.13 mg 5.32 mg 38 % Phosphorous 657.69 mg 162.13 mg 405.33 mg 58 % Magnesium 92.31 mg 21.96 mg 54.90 mg 15 % Zinc 14.23 mg 1.86 mg 4.65 mg 47 % Iodine 153.85 µg 19.40 µg 48.50 µg 32 % -

Know Your Serving Size.Pub
KNOW YOUR SERVING SIZE Servings in restaurants, coffee shops and Eating Well with Canada’s Food Guide supermarkets are getting larger and larger. identifies what a healthy serving size is It is no wonder why so many people are making it is easy to plan meals. confused about what a healthy serving size is. We are continuously bombarded with Figuring out the Food Guide servings will extra large servings such as super-size or take time. Measuring food is more accurate biggie size which appear to save us money. but is not always convenient. Estimating a However, in the long run, our bodies are Food Guide serving can be as simple as paying for it. As serving sizes grow so do using visual aides like the palm of your our waist lines! Knowing what a healthy hand, a tennis ball or golf ball. The serving serving size looks like will help you put an size guide will help you visualize a serving end to portion distortion. size. What more can I do? You don’t have to “clean your plate”... Also consider: Eating just so your plate is clean is an idea • Purchasing single portion snacks to that you should try to abandon. Start avoid eating more than one with smaller portions, pace yourself and • Eating snacks from a bowl instead of enjoy the taste of your food. Listen to out of a bag your body and stop when you feel • Having clean, cut up fruits or vegetables satisfied. Save the leftovers for the next ready in your fridge for snacking or day when you can enjoy the same great meals taste all over again. -

Food Label Tip: Watch Your Calories and Serving Sizes
Food Label Tip: Watch Your Calories and Serving Sizes It is important to watch your calories for weight control. To do this, you need to know how calories and serving size are related. This will help you choose foods and decide what amounts to eat or drink to keep a healthy weight. Learn about calories and serving size by looking at these food labels: Nutrition Facts Nutrition Facts Serving Size 1/2 cup (110g) Serving Size 1/2 cup (110g) Servings Per Container 3.5 Servings Per Container 3.5 Amount Per Serving Amount Per Serving Calories 50 Calories from Fat 0 Calories 100 Calories from Fat 0 % Daily Value* % Daily Value* Total Fat 0g 0% Total Fat 0g 0% Saturated Fat 0g 0% Saturated Fat 0g 0% In Fruit Juice Trans Fat 0g Trans Fat 0g In Heavy Syrup Cholesterol 0mg 0% Cholesterol 0mg 0% Sodium 25mg 1% Sodium 10mg 0% Total Carbohydrate 13g 4% Total Carbohydrate 24g 8% Dietary Fiber 1g 4% Dietary Fiber 1g 4% Sugars 9g Sugars 23g Protein less than 1g Protein 0g * Percent Daily Values are based on a 2,000 calorie diet. Vitamin A 8% Vitamin C 2% Vitamin A 2% Vitamin C 2% Your Daily Values may be higher or lower depending on Calcium 0% Iron 0% Calcium 0% Iron 0% your calorie needs. This is where you find the Here is where you find calories on a food label. There are “Serving Size” on a food label. 50 calories in 1 serving (1/2 cup) of canned peaches in The Serving Size for both types fruit juice. -

Fish Technology Glossary
Glossary of Fish Technology Terms A Selection of Terms Compiled by Kevin J. Whittle and Peter Howgate Prepared under contract to the Fisheries Industries Division of the Food and Agriculture Organization of the United Nations 6 December 2000 Last updated: February 2002 Kevin J. Whittle 1 GLOSSARY OF FISH TECHNOLOGY TERMS [Words highlighted in bold in the text of an entry refer to another entry. Words in parenthesis are alternatives.] Abnormalities Attributes of the fish that are not found in the great majority of that kind of fish. For example: atypical shapes; overall or patchy discolorations of skin or of fillet; diseased conditions; atypical odours or flavours. Generally, the term should be used for peculiarities present in the fish at the time of capture or harvesting, or developing very soon after; peculiarities arising during processing should be considered as defects. Acetic acid Formal chemical name, ethanoic acid. An organic acid of formula CH3.COOH. It is the main component, 3-6%, other than water, of vinegar. Used in fish technology in preparation of marinades. Acid curing See Marinating Actomyosin A combination of the two main proteins, actin and myosin, present in all muscle tissues. Additive A chemical added to a food to affect its properties. Objectives of including additives in a product include: increased stability during storage; inhibition of growth of microorganisms or production of microbial toxins; prevention or reduction of formation of off-flavours; improved sensory properties, particularly colours and appearance, affecting acceptability to the consumer; improved properties related to preparation and processing of food, for example, ability to create stable foams or emulsions, or to stabilise or thicken sauces. -
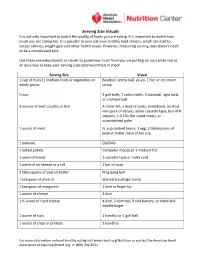
Serving Size Visuals It Is Not Only Important to Watch the Quality of Foods You Are Eating, It Is Important to Watch How Much You Are Eating Too
Serving Size Visuals It is not only important to watch the quality of foods you are eating, it is important to watch how much you are eating too. It is possible to over-eat even healthy food choices, which can lead to excess calories, weight gain and other health issues. However, measuring serving sizes doesn’t need to be a complicated task. Use these everyday objects as visuals to guide how much food you are putting on your plate and as an easy way to keep your serving sizes (and waistline!) in check. Serving Size Visual 1 cup of fruits (1 medium fruit) or vegetables or Baseball, tennis ball, yo-yo, 1 fist, or ice cream whole grains scoop ½ cup 2 golf balls, 7 cotton balls, ½ baseball, light bulb, or a billiard ball 3 ounces of beef, poultry or fish A dollar bill, a deck of cards, checkbook, an iPod, mini pack of tissues, audio cassette tape, box of 8 crayons, 2-3 CDs (for sliced meat), or outstretched palm 1 ounce of meat ½ cup cooked beans, 1 egg, 2 tablespoons of peanut butter, base of tea cup 1 pancake CD/DVD 1 baked potato Computer mouse or 1 medium fist 1 piece of bread 1 cassette tape or index card 1 piece of cornbread or a roll 1 bar of soap 2 tablespoons of peanut butter Ping pong ball 1 teaspoon of olive oil Standard postage stamp 1 teaspoon of margarine 1 dice or finger tip 1 ounce of cheese 3 dice 1 ½ ounce of hard cheese 4 dice, 3 dominos, 9 volt battery, or index and middle finger 1 ounce of nuts 1 handful or 1 golf ball 1 ounce of chips or pretzels 2 handfuls For more information on heart-healthy eating visit www.Heart.org/Nutrition or contact the American Heart Association at [email protected] or (800) 242-8721. -

Δtb = M × Kb, Δtf = M × Kf
8.1HW Colligative Properties.doc Colligative Properties of Solvents Use the Equations given in your notes to solve the Colligative Property Questions. ΔTb = m × Kb, ΔTf = m × Kf Freezing Boiling K K Solvent Formula Point f b Point (°C) (°C/m) (°C/m) (°C) Water H2O 0.000 100.000 1.858 0.521 Acetic acid HC2H3O2 16.60 118.5 3.59 3.08 Benzene C6H6 5.455 80.2 5.065 2.61 Camphor C10H16O 179.5 ... 40 ... Carbon disulfide CS2 ... 46.3 ... 2.40 Cyclohexane C6H12 6.55 80.74 20.0 2.79 Ethanol C2H5OH ... 78.3 ... 1.07 1. Which solvent’s freezing point is depressed the most by the addition of a solute? This is determined by the Freezing Point Depression constant, Kf. The substance with the highest value for Kf will be affected the most. This would be Camphor with a constant of 40. 2. Which solvent’s freezing point is depressed the least by the addition of a solute? By the same logic as above, the substance with the lowest value for Kf will be affected the least. This is water. Certainly the case could be made that Carbon disulfide and Ethanol are affected the least as they do not have a constant. 3. Which solvent’s boiling point is elevated the least by the addition of a solute? Water 4. Which solvent’s boiling point is elevated the most by the addition of a solute? Acetic Acid 5. How does Kf relate to Kb? Kf > Kb (fill in the blank) The freezing point constant is always greater. -

Disrupting Crystalline Order to Restore Superfluidity
Abteilung Kommunikation und Öffentlichkeitsarbeit Referat Medien- und Öffentlichkeitsarbeit Tel. +49 40 42838-2968 Fax +49 40 42838-2449 E-Mail: [email protected] 12. Oktober 2018 Pressedienst 57/18 Disrupting crystalline order to restore superfluidity When we put water in a freezer, water molecules crystallize and form ice. This change from one phase of matter to another is called a phase transition. While this transition, and countless others that occur in nature, typically takes place at the same fixed conditions, such as the freezing point, one can ask how it can be influenced in a controlled way. We are all familiar with such control of the freezing transition, as it is an essential ingredient in the art of making a sorbet or a slushy. To make a cold and refreshing slushy with the perfect consistency, constant mixing of the liquid is needed. For example, a slush machine with constantly rotating blades helps prevent water molecules from crystalizing and turning the slushy into a solid block of ice. Imagine now controlling quantum matter in this same way. Rather than forming a normal liquid, like a melted slushy under the sun for too long, quantum matter can form a superfluid. This mysterious and counterintuitive form of matter was first observed in liquid helium at very low temperatures, less than 2 Kelvin above absolute zero. The helium atoms have a strong tendency to form a crystal, like the water molecules in a slushy, and this restricts the superfluid state of helium to very low temperatures and low pressures. But what if you could turn on the blades in your slush machine for quantum matter? What if you could disrupt the crystalline order so that the superfluid could flow freely even at temperatures and pressures where it usually does not? This is indeed the idea that was demonstrated by a team of scientists led by Ludwig Mathey and Andreas Hemmerich from the University of Hamburg. -

Physical Changes
How Matter Changes By Cindy Grigg Changes in matter happen around you every day. Some changes make matter look different. Other changes make one kind of matter become another kind of matter. When you scrunch a sheet of paper up into a ball, it is still paper. It only changed shape. You can cut a large, rectangular piece of paper into many small triangles. It changed shape and size, but it is still paper. These kinds of changes are called physical changes. Physical changes are changes in the way matter looks. Changes in size and shape, like the changes in the cut pieces of paper, are physical changes. Physical changes are changes in the size, shape, state, or appearance of matter. Another kind of physical change happens when matter changes from one state to another state. When water freezes and makes ice, it is still water. It has only changed its state of matter from a liquid to a solid. It has changed its appearance and shape, but it is still water. You can change the ice back into water by letting it melt. Matter looks different when it changes states, but it stays the same kind of matter. Solids like ice can change into liquids. Heat speeds up the moving particles in ice. The particles move apart. Heat melts ice and changes it to liquid water. Metals can be changed from a solid to a liquid state also. Metals must be heated to a high temperature to melt. Melting is changing from a solid state to a liquid state. -

A Possible Force Mechanism Associated with the Freezing of Water in Porous Materials
A Possible Force Mechanism Associated with the Freezing of Water in Porous Materials LORNE W. GOLD Division of Building Research, National Research Council, Ottawa, Canada The thermodynamic equilibrium conditions for a water-ice interface in a pore of a porous medium are derived. It is found that, taking the geometry and physical dimensions of the pore into consideration, a positive pressure must develop between the ice and the solid in order for equilibrium to be maintained when the temperature at the freezing plane is depressed. A simple model utilizing this mecha• nism exhibits the same properties as a frost-heaving soil. The in• fluence of air in the pores is briefly discussed. #WHEN WATER is present in porous materials and is allowed to freeze, a force may be developed which is not directly related to the expansion that occurs when water changes to ice. Although many observations have been made on this phenomenon, par• ticularly in the field of soil mechanics, a satisfactory explanation of the origin of this force has not appeared. Experiments to date indicate how the magnitude of this force depends on certain physical characteristics of the system. The following are some of the facts observed during experiments that a theory on the origin of this force must explain: 1. During the freezing process, the freezing plane may remain stationary while the ice phase continues to grow. When this occurs, the frozen portion is displaced rela• tive to the unfrozen and a layer of ice, often called an ice lens is formed. The dis• placement may be microscopic or it may amount to several inches. -

Portion Distortion Written by Joseph Ewing, RD, LDN
Portion Distortion Written by Joseph Ewing, RD, LDN Over the past several years, portion sizes have increased significantly! With this increase in portion sizes, there has been a noticeable increase in obesity in people of all ages and ethnicities. Portion distortion refers to what “we” feel is a “normal” portion size versus what is actually being served. For example, a standard portion of steak is 3 ounces… Have you ever gone to a restaurant and ordered a 3 ounce steak? No, because it is not available. Most times when order a steak they come in 8 ounces, 12 ounces, 24 ounces or more! That’s incredible! We’re talking about an astronomical calorie difference. The recommended 3oz Filet Mignon has 130 calories vs. an 8 ounce filet mignon with 301 calories (served at Outback Steakhouse (www.caloriecount.com)). Portion vs. Serving Image: milesforthought.wordpress.com According to the National Institutes of Health, a “portion” is how much food you choose to eat at one time, whether it is at home, in a restaurant, prepackaged or homemade. A “serving” size is a specific amount of food or beverage such as 1 cup of pudding or 1 slice of bread. Sometimes when you are eating a “portion” of food, it matches the serving size, but many times it does not. In order to explain this concept, let’s look at a can of Campbell’s Condensed Tomato Soup. Serving size is ½ cup Servings per container are about 2.5. This means if you eat this whole can of soup you would be consuming 2.5 servings of soup! If you consumed 1 (1/2 cup) portion of this soup, you would consume 480mg of If you consumed the sodium. -

Understanding Freezing Behavior in Nano-Porous Materials: Free
ÍÒÖ×ØÒÒ ÖÞÒ ÚÓÖ Ò ÆÒÓ¹Ô ÓÖÓÙ× ÅØÖÐ× Ö ÒÖ Ý ËÑÙÐØÓÒ ËØÙ× Ò ÜÔ ÖÑÒØ ××ÖØØÓÒ ÈÖ×ÒØ ØÓ Ø ÙÐØÝ Ó Ø Ö ÙØ Ë Ó ÓÐ Ó ÓÖÒÐÐ ÍÒÚÖ×ØÝ Ò ÈÖØÐ ÙЬÐÐÑÒØ Ó Ø ÊÕÙÖÑÒØ× ÓÖ Ø Ö Ó Ó ØÓÖ Ó È ÐÓ×ÓÔ Ý Ý ÊÚ ÊÖ×ÒÒ ÙÙ×Ø ¾¼¼¼ ÊÚ ÊÖ×ÒÒ ¾¼¼¼ ÄÄ ÊÁÀÌË ÊËÊÎ ÍÒÖ×ØÒÒ ÖÞÒ ÚÓÖ Ò ÆÒÓ¹Ô ÓÖÓÙ× ÅØÖÐ× Ö ÒÖÝ ËÑÙÐØÓÒ ËØÙ× Ò ÜÔ ÖÑÒØ ÊÚ ÊÖ×ÒÒ¸ Ⱥº ÓÖÒÐÐ ÍÒÚÖ×ØÝ ¾¼¼¼ ÍÒÖ×ØÒÒ Ô× ÚÓÖ Ò ÓÒ¬Ò ×Ý×ØÑ× × ØÖÑÒÓÙ× Ô ÓØÒØÐ Ò ÑÔÖÓÚÒ Ø Æ ÓÚÖØÝ Ó ×ÔÖØÓÒ ÔÖÓ ××× ØØ Ù× Ô ÓÖÓÙ× Ñ¹ ØÖÐ× Ð ØÚØ Ö ÓÒ׸ ÓÒØÖÓÐÐ Ð××׸ ×Ð ÜÖÓÐ× ² ÖÓÐ׸ Ö ÓÒ ÖÓÐ× Ò ÞÓÐØ׺ Ì «Ø× Ó Ø ÖÙ ÑÒ×ÓÒÐØÝ Ò ÒÒ ÒÖ¹ Ø ÒØÖØÓÒ× Ù ØÓ Ø ÔÓÖÓÙ× ×ÙÖ Ú ÑÔ ÓÖØÒØ ÓÒ×ÕÙÒ× ØØ Ò ÓÒÐÝ ÔØÙÖ Ý ÑÓÐÙÐÖ ÐÚÐ ÑÓ ÐÒº ÆÓÚÐ Ô× ØÖÒ×ØÓÒ× Ò Ö×ÙÐØ ´ÔÐÐÖÝ ÓÒÒ×ØÓÒ Ò ÕÙ×¹ÓÒ¹ÑÒ×ÓÒÐ ×Ý×ØÑ× Ò ÓÖÒØØÓÒÐ ÓÖÖÒ ØÖÒ×ØÓÒ× Ò ÕÙ×¹ØÛÓ¹ÑÒ×ÓÒÐ ×Ý×ØÑ×µ ØØ ÓØÒ Ö Ø Ù× Ó Ø Ö¹ ÓÛÒ Ó ÑÖÓ×ÓÔ ÕÙØÓÒ× Ð ÃÐÚÒ Ò ×¹ÌÓÑ×ÓÒ ÕÙØÓÒ× × ÓÒ Ð××Ð ØÖÑÓ ÝÒÑ׺ ÁÒ Ø× ÛÓÖ¸ Ø Ó Ù× ÓÒ Ö ÒÖÝ ÑØÓ × ÓÒ ÄÒÙ ØÓÖÝ Û Û ÚÐÓÔ Ò ÔÔÐØÓÒ× Ó Ø× ØÓÖÝ ØÓ ÙÒÖ×ØÒ Ø ÖÓÛÒ Ó Ø ÑÖÓ×ÓÔ ÕÙØÓÒ׸ ÚÐÓÔÒ ÐÓÐ Ô× ÖÑ׸ Ò ÖØÖÞÒ ÜØ Ô×׺ Ì Ö×ÙÐØ× Ó ÓÙÖ ÜÔ ÖÑÒØÐ ×ØÙ× Ö Ð×Ó ×Ö ¸ ØØ ÔÖÓÚ ×ÙÔÔ ÓÖØÒ ÚÒ ÓÖ ÓÙÖ ÑÓ ÐÒ «ÓÖØ׺ ÓÖÔÐ Ë Ø ÓÖÒ Ò Ò Ñ ÑÐÝ Ò ×ÓÙØÖÒ ÁÒ¸ Á Û× ÒØÖ×Ø Ò Ö×Ö ×Ò Ø Ý× Á Ò³Ø ÚÒ ÖÑÑÖº Á ÓÙÒ ÑÝ×Ð ´ÖØÖ ÓÖØÙÒØÐÝ ÓÖ Ñµ ×ØÙÝÒ ÑÐ ÒÒÖÒ Ò Ø ÁÒÒ ÁÒ×ØØÙØ Ó ÌÒÓÐÓÝ Ø ÅÖ׺ ÙÖÒ ÑÝ Ö×ÑÒ ÝÖ Á Û× ×ØÖÙ Ý Ø ÖÐÞØÓÒ ØØ Á Ñ ÒÓ ÐÓÒÖ Ø ÒÓÛ¹ÐÐ Ô Ö×ÓÒ Ò ×Ó Óи ØØ Ø ÛÓÖÐ × ÙÐÐ Ó ÔÝ×Ð ´Ò Ñе ÔÒÓÑÒ ØØ Á ÖÐÝ ÒÛ ÓÙغ Ø Ø ÒÒÒ Ó ÑÝÂÙÒÓÖÝÖ¸ Á ×ØÖØ ØÓ ÚÐÓÔ Ò ÒØÒ× ×ÒØÓÒ ÓÖ ×ØØ×ØÐ ÑÒ× Ò Ò ÑÝ ÑÒ ØÓ ÕÙØ ÑÐ ÒÒÖÒ Ò Ø ÙÔ ÔÝ×׺ ÁØ Û× Ô× ÛÖ Á Û× ÑÒ Ø ÓÒØÒØ× Ó ÑÒÝ Ó Ó× ÓÒ ×ØØ×ØÐ ÔÝ×׸ ÛÒ Á Ñ ÖÓ×× Ø ÓÓ ÅÓÐÙÐÖ ÌÖÑÓ ÝÒÑ× Ó ÐÙ È× ÕÙÐÖ Ý ÂÓÒ ÈÖÙ×ÒØÞº Ì× Û× ØÙÖÒÒ -

Meeting the NSLP New Meal Pattern
We Can Do This: Advice and Resources for Meeting the NSLP New Meal Pattern as of December 2012 Purpose: School Nutrition Professionals are in the process of implementing the new USDA Meal Pattern and have asked for SNA to provide easy- to-find answers to their Meal Pattern questions as well as sample menus and tools from States and local school districts that will help others not have to “reinvent the wheel.” For the entire “We Can Do This: Advice and Resources for Meeting NSLP New Meal Pattern,” visit www.schoolnutrition.org/mealpattern. Contents: This Resource Guide will be updated as new guidance and additional examples become available. USDA Frequently Asked Questions (FAQ’s) are incorporated in the appropriate chapter(s). This Guide covers Lunch Programs only. Ad Hoc Committee: Colleen Fillmore, SNS Pat McCoy, SNS U.S. Department of Agriculture Director of Child Nutrition Vice President, Field Sales Washington, DC/Alexandria, VA Idaho Department of Education Schwan’s Food Service, Inc. Boise, ID Marshall, MN Project Consultant: Wanda Grant, SNS Katie Millett George Sneller (retired) Child Nutrition Director Director Jacksonville, FL Palm Springs Unified School District MA Department of Elementary and Secondary Former Child Nutrition Director Palm Springs, CA Education Office of Superintendent of Public Instruction Abington, MA Washington State Catherine Digilio Grimes, SNS State Director, School Nutrition Programs Jean Ronnei, SNS SNA Staff: Virginia Department of Education Director, Nutrition & Custodial Services Cathy Schuchart