Physical Chemistry Laboratory I CHEM 445 Experiment 1 Freezing Point Depression of Electrolytes Revised, 01/13/03
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Solutes and Solution
Solutes and Solution The first rule of solubility is “likes dissolve likes” Polar or ionic substances are soluble in polar solvents Non-polar substances are soluble in non- polar solvents Solutes and Solution There must be a reason why a substance is soluble in a solvent: either the solution process lowers the overall enthalpy of the system (Hrxn < 0) Or the solution process increases the overall entropy of the system (Srxn > 0) Entropy is a measure of the amount of disorder in a system—entropy must increase for any spontaneous change 1 Solutes and Solution The forces that drive the dissolution of a solute usually involve both enthalpy and entropy terms Hsoln < 0 for most species The creation of a solution takes a more ordered system (solid phase or pure liquid phase) and makes more disordered system (solute molecules are more randomly distributed throughout the solution) Saturation and Equilibrium If we have enough solute available, a solution can become saturated—the point when no more solute may be accepted into the solvent Saturation indicates an equilibrium between the pure solute and solvent and the solution solute + solvent solution KC 2 Saturation and Equilibrium solute + solvent solution KC The magnitude of KC indicates how soluble a solute is in that particular solvent If KC is large, the solute is very soluble If KC is small, the solute is only slightly soluble Saturation and Equilibrium Examples: + - NaCl(s) + H2O(l) Na (aq) + Cl (aq) KC = 37.3 A saturated solution of NaCl has a [Na+] = 6.11 M and [Cl-] = -

Prediction of Osmotic Coefficients by Pair Correlation Function Method" (1991)
New Jersey Institute of Technology Digital Commons @ NJIT Dissertations Electronic Theses and Dissertations Spring 1-31-1991 Thermodynamics of strong electrolyte solutions : prediction of osmotic coefficientsy b pair correlation function method One Kwon Rim New Jersey Institute of Technology Follow this and additional works at: https://digitalcommons.njit.edu/dissertations Part of the Chemical Engineering Commons Recommended Citation Rim, One Kwon, "Thermodynamics of strong electrolyte solutions : prediction of osmotic coefficients by pair correlation function method" (1991). Dissertations. 1145. https://digitalcommons.njit.edu/dissertations/1145 This Dissertation is brought to you for free and open access by the Electronic Theses and Dissertations at Digital Commons @ NJIT. It has been accepted for inclusion in Dissertations by an authorized administrator of Digital Commons @ NJIT. For more information, please contact [email protected]. Copyright Warning & Restrictions The copyright law of the United States (Title 17, United States Code) governs the making of photocopies or other reproductions of copyrighted material. Under certain conditions specified in the law, libraries and archives are authorized to furnish a photocopy or other reproduction. One of these specified conditions is that the photocopy or reproduction is not to be “used for any purpose other than private study, scholarship, or research.” If a, user makes a request for, or later uses, a photocopy or reproduction for purposes in excess of “fair use” that user may be -

Solution Is a Homogeneous Mixture of Two Or More Substances in Same Or Different Physical Phases. the Substances Forming The
CLASS NOTES Class:12 Topic: Solutions Subject: Chemistry Solution is a homogeneous mixture of two or more substances in same or different physical phases. The substances forming the solution are called components of the solution. On the basis of number of components a solution of two components is called binary solution. Solute and Solvent In a binary solution, solvent is the component which is present in large quantity while the other component is known as solute. Classification of Solutions Solubility The maximum amount of a solute that can be dissolved in a given amount of solvent (generally 100 g) at a given temperature is termed as its solubility at that temperature. The solubility of a solute in a liquid depends upon the following factors: (i) Nature of the solute (ii) Nature of the solvent (iii) Temperature of the solution (iv) Pressure (in case of gases) Henry’s Law The most commonly used form of Henry’s law states “the partial pressure (P) of the gas in vapour phase is proportional to the mole fraction (x) of the gas in the solution” and is expressed as p = KH . x Greater the value of KH, higher the solubility of the gas. The value of KH decreases with increase in the temperature. Thus, aquatic species are more comfortable in cold water [more dissolved O2] rather than Warm water. Applications 1. In manufacture of soft drinks and soda water, CO2 is passed at high pressure to increase its solubility. 2. To minimise the painful effects (bends) accompanying the decompression of deep sea divers. O2 diluted with less soluble. -

Thermodynamic Properties of 1:1 Salt Aqueous Solutions with the Electrolattice M
D o s s i e r This paper is a part of the hereunder thematic dossier published in OGST Journal, Vol. 68, No. 2, pp. 187-396 and available online here Cet article fait partie du dossier thématique ci-dessous publié dans la revue OGST, Vol. 68, n°2, pp. 187-396 et téléchargeable ici © Photos: IFPEN, Fotolia, X. DOI: 10.2516/ogst/2012094 DOSSIER Edited by/Sous la direction de : Jean-Charles de Hemptinne InMoTher 2012: Industrial Use of Molecular Thermodynamics InMoTher 2012 : Application industrielle de la thermodynamique moléculaire Oil & Gas Science and Technology – Rev. IFP Energies nouvelles, Vol. 68 (2013), No. 2, pp. 187-396 Copyright © 2013, IFP Energies nouvelles 187 > Editorial électrostatique ab initio X. Rozanska, P. Ungerer, B. Leblanc and M. Yiannourakou 217 > Improving the Modeling of Hydrogen Solubility in Heavy Oil Cuts Using an Augmented Grayson Streed (AGS) Approach 309 > Improving Molecular Simulation Models of Adsorption in Porous Materials: Modélisation améliorée de la solubilité de l’hydrogène dans des Interdependence between Domains coupes lourdes par l’approche de Grayson Streed Augmenté (GSA) Amélioration des modèles d’adsorption dans les milieux poreux R. Torres, J.-C. de Hemptinne and I. Machin par simulation moléculaire : interdépendance entre les domaines J. Puibasset 235 > Improving Group Contribution Methods by Distance Weighting Amélioration de la méthode de contribution du groupe en pondérant 319 > Performance Analysis of Compositional and Modified Black-Oil Models la distance du groupe For a Gas Lift Process A. Zaitseva and V. Alopaeus Analyse des performances de modèles black-oil pour le procédé d’extraction par injection de gaz 249 > Numerical Investigation of an Absorption-Diffusion Cooling Machine Using M. -

Dissociation Constants and Ph-Titration Curves at Constant Ionic Strength from Electrometric Titrations in Cells Without Liquid
U. S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS RESEARCH PAPER RP1537 Part of Journal of Research of the N.ational Bureau of Standards, Volume 30, May 1943 DISSOCIATION CONSTANTS AND pH-TITRATION CURVES AT CONSTANT IONIC STRENGTH FROM ELECTRO METRIC TITRATIONS IN CELLS WITHOUT LIQUID JUNCTION : TITRATIONS OF FORMIC ACID AND ACETIC ACID By Roger G. Bates, Gerda L. Siegel, and S. F. Acree ABSTRACT An improved method for obtaining the titration curves of monobasic acids is outlined. The sample, 0.005 mole of the sodium salt of the weak acid, is dissolver! in 100 ml of a 0.05-m solution of sodium chloride and titrated electrometrically with an acid-salt mixture in a hydrogen-silver-chloride cell without liquid junction. The acid-salt mixture has the composition: nitric acid, 0.1 m; pot assium nitrate, 0.05 m; sodium chloride, 0.05 m. The titration therefore is performed in a. medium of constant chloride concentration and of practically unchanging ionic strength (1'=0.1) . The calculations of pH values and of dissociation constants from the emf values are outlined. The tit ration curves and dissociation constants of formic acid and of acetic acid at 25 0 C were obtained by this method. The pK values (negative logarithms of the dissociation constants) were found to be 3.742 and 4. 754, respectively. CONTENTS Page I . Tntroduction __ _____ ~ __ _______ . ______ __ ______ ____ ________________ 347 II. Discussion of the titrat ion metbod __ __ ___ ______ _______ ______ ______ _ 348 1. Ti t;at~on. clU,:es at constant ionic strength from cells without ltqUld JunctlOlL - - - _ - __ _ - __ __ ____ ____ _____ __ _____ ____ __ _ 349 2. -

Ideal Gas Law PV = Nrt R = Universal Gas Constant R = 0.08206 L-Atm R = 8.314 J Mol-K Mol-K Example
Ideal gas law PV = nRT R = universal gas constant R = 0.08206 L-atm R = 8.314 J mol-K mol-K Example: In the reaction of oxygen with carbon to produce carbon dioxide, how many liters of oxygen at 747 mm Hg and 21.0 °C are needed to react with 24.022 g of carbon? CHEM 102 Winter 2011 Practice: In the reaction of oxygen and hydrogen to make water, how many liters of hydrogen at exactly 0 °C and 1 atm are needed to react with 10.0 g of oxygen? 2 CHEM 102 Winter 2011 Combined gas law V decreases with pressure (V α 1/P). V increases with temperature (V α T). V increases with particles (V α n). Combining these ideas: V α n x T P For a given amount of gas (constant number of particles, ninitial = nafter) that changes in volume, temperature, or pressure: Vinitial α ninitial x Tinitial Pinitial Vafter α nafter x Tafter Pafter ninitial = n after Vinitial x Pinitial = Vafter x Pafter Tinitial Tafter 3 CHEM 102 Winter 2011 Example: A sample of gas is pressurized to 1.80 atm and volume of 90.0 cm3. What volume would the gas have if it were pressurized to 4.00 atm without a change in temperature? 4 CHEM 102 Winter 2011 Example: A 2547-mL sample of gas is at 421 °C. What is the new gas temperature if the gas is cooled at constant pressure until it has a volume of 1616 mL? 5 CHEM 102 Winter 2011 Practice: A balloon is filled with helium. -

Ideal Gasses Is Known As the Ideal Gas Law
ESCI 341 – Atmospheric Thermodynamics Lesson 4 –Ideal Gases References: An Introduction to Atmospheric Thermodynamics, Tsonis Introduction to Theoretical Meteorology, Hess Physical Chemistry (4th edition), Levine Thermodynamics and an Introduction to Thermostatistics, Callen IDEAL GASES An ideal gas is a gas with the following properties: There are no intermolecular forces, except during collisions. All collisions are elastic. The individual gas molecules have no volume (they behave like point masses). The equation of state for ideal gasses is known as the ideal gas law. The ideal gas law was discovered empirically, but can also be derived theoretically. The form we are most familiar with, pV nRT . Ideal Gas Law (1) R has a value of 8.3145 J-mol1-K1, and n is the number of moles (not molecules). A true ideal gas would be monatomic, meaning each molecule is comprised of a single atom. Real gasses in the atmosphere, such as O2 and N2, are diatomic, and some gasses such as CO2 and O3 are triatomic. Real atmospheric gasses have rotational and vibrational kinetic energy, in addition to translational kinetic energy. Even though the gasses that make up the atmosphere aren’t monatomic, they still closely obey the ideal gas law at the pressures and temperatures encountered in the atmosphere, so we can still use the ideal gas law. FORM OF IDEAL GAS LAW MOST USED BY METEOROLOGISTS In meteorology we use a modified form of the ideal gas law. We first divide (1) by volume to get n p RT . V we then multiply the RHS top and bottom by the molecular weight of the gas, M, to get Mn R p T . -

Physical Chemistry
Subject Chemistry Paper No and Title 10, Physical Chemistry- III (Classical Thermodynamics, Non-Equilibrium Thermodynamics, Surface chemistry, Fast kinetics) Module No and Title 10, Free energy functions and Partial molar properties Module Tag CHE_P10_M10 CHEMISTRY Paper No. 10: Physical Chemistry- III (Classical Thermodynamics, Non-Equilibrium Thermodynamics, Surface chemistry, Fast kinetics) Module No. 10: Free energy functions and Partial molar properties TABLE OF CONTENTS 1. Learning outcomes 2. Introduction 3. Free energy functions 4. The effect of temperature and pressure on free energy 5. Maxwell’s Relations 6. Gibbs-Helmholtz equation 7. Partial molar properties 7.1 Partial molar volume 7.2 Partial molar Gibb’s free energy 8. Question 9. Summary CHEMISTRY Paper No. 10: Physical Chemistry- III (Classical Thermodynamics, Non-Equilibrium Thermodynamics, Surface chemistry, Fast kinetics) Module No. 10: Free energy functions and Partial molar properties 1. Learning outcomes After studying this module you shall be able to: Know about free energy functions i.e. Gibb’s free energy and work function Know the dependence of Gibbs free energy on temperature and pressure Learn about Gibb’s Helmholtz equation Learn different Maxwell relations Derive Gibb’s Duhem equation Determine partial molar volume through intercept method 2. Introduction Thermodynamics is used to determine the feasibility of the reaction, that is , whether the process is spontaneous or not. It is used to predict the direction in which the process will be spontaneous. Sign of internal energy alone cannot determine the spontaneity of a reaction. The concept of entropy was introduced in second law of thermodynamics. Whenever a process occurs spontaneously, then it is considered as an irreversible process. -
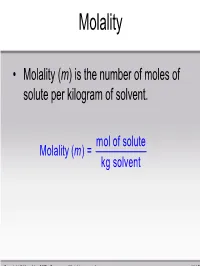
Mol of Solute Molality ( ) = Kg Solvent M
Molality • Molality (m) is the number of moles of solute per kilogram of solvent. mol of solute Molality (m ) = kg solvent Copyright © Houghton Mifflin Company. All rights reserved. 11 | 51 Sample Problem Calculate the molality of a solution of 13.5g of KF dissolved in 250. g of water. mol of solute m = kg solvent 1 m ol KF ()13.5g 58.1 = 0.250 kg = 0.929 m Copyright © Houghton Mifflin Company. All rights reserved. 11 | 52 Mole Fraction • Mole fraction (χ) is the ratio of the number of moles of a substance over the total number of moles of substances in solution. number of moles of i χ = i total number of moles ni = nT Copyright © Houghton Mifflin Company. All rights reserved. 11 | 53 Sample Problem -Conversions between units- • ex) What is the molality of a 0.200 M aluminum nitrate solution (d = 1.012g/mL)? – Work with 1 liter of solution. mass = 1012 g – mass Al(NO3)3 = 0.200 mol × 213.01 g/mol = 42.6 g ; – mass water = 1012 g -43 g = 969 g 0.200mol Molality==0.206 mol / kg 0.969kg Copyright © Houghton Mifflin Company. All rights reserved. 11 | 54 Sample Problem Calculate the mole fraction of 10.0g of NaCl dissolved in 100. g of water. mol of NaCl χ=NaCl mol of NaCl + mol H2 O 1 mol NaCl ()10.0g 58.5g NaCl = 1 m ol NaCl 1 m ol H2 O ()10.0g+ () 100.g H2 O 58.5g NaCl 18.0g H2 O = 0.0299 Copyright © Houghton Mifflin Company. -

Simple Mixtures
Simple mixtures Before dealing with chemical reactions, here we consider mixtures of substances that do not react together. At this stage we deal mainly with binary mixtures (mixtures of two components, A and B). We therefore often be able to simplify equations using the relation xA + xB = 1. A restriction in this chapter – we consider mainly non-electrolyte solutions – the solute is not present as ions. The thermodynamic description of mixtures We already considered the partial pressure – the contribution of one component to the total pressure, when we were dealing with the properties of gas mixtures. Here we introduce other analogous partial properties. Partial molar quantities: describe the contribution (per mole) that a substance makes to an overall property of mixture. The partial molar volume, VJ – the contribution J makes to the total volume of a mixture. Although 1 mol of a substance has a characteristic volume when it is pure, 1 mol of a substance can make different contributions to the total volume of a mixture because molecules pack together in different ways in the pure substances and in mixtures. Imagine a huge volume of pure water. When a further 1 3 mol H2O is added, the volume increases by 18 cm . When we add 1 mol H2O to a huge volume of pure ethanol, the volume increases by only 14 cm3. 18 cm3 – the volume occupied per mole of water molecules in pure water. 14 cm3 – the volume occupied per mole of water molecules in virtually pure ethanol. The partial molar volume of water in pure water is 18 cm3 and the partial molar volume of water in pure ethanol is 14 cm3 – there is so much ethanol present that each H2O molecule is surrounded by ethanol molecules and the packing of the molecules results in the water molecules occupying only 14 cm3. -

Chapter 9 Ideal and Real Solutions
2/26/2016 CHAPTER 9 IDEAL AND REAL SOLUTIONS • Raoult’s law: ideal solution • Henry’s law: real solution • Activity: correlation with chemical potential and chemical equilibrium Ideal Solution • Raoult’s law: The partial pressure (Pi) of each component in a solution is directly proportional to the vapor pressure of the corresponding pure substance (Pi*) and that the proportionality constant is the mole fraction (xi) of the component in the liquid • Ideal solution • any liquid that obeys Raoult’s law • In a binary liquid, A-A, A-B, and B-B interactions are equally strong 1 2/26/2016 Chemical Potential of a Component in the Gas and Solution Phases • If the liquid and vapor phases of a solution are in equilibrium • For a pure liquid, Ideal Solution • ∆ ∑ Similar to ideal gas mixing • ∆ ∑ 2 2/26/2016 Example 9.2 • An ideal solution is made from 5 mole of benzene and 3.25 mole of toluene. (a) Calculate Gmixing and Smixing at 298 K and 1 bar. (b) Is mixing a spontaneous process? ∆ ∆ Ideal Solution Model for Binary Solutions • Both components obey Rault’s law • Mole fractions in the vapor phase (yi) Benzene + DCE 3 2/26/2016 Ideal Solution Mole fraction in the vapor phase Variation of Total Pressure with x and y 4 2/26/2016 Average Composition (z) • , , , , ,, • In the liquid phase, • In the vapor phase, za x b yb x c yc Phase Rule • In a binary solution, F = C – p + 2 = 4 – p, as C = 2 5 2/26/2016 Example 9.3 • An ideal solution of 5 mole of benzene and 3.25 mole of toluene is placed in a piston and cylinder assembly. -

Molar Gas Constant
Molar gas constant The molar gas constant (also known as the universal or ideal gas constant and designated by the symbol R ) is an important physical constant which appears in many of the fundamental equations inphysics, chemistry and engineering, such as the ideal gas law, other equations of state and the Nernst equation. R is equivalent to the Boltzmann constant (designated kB) times Avogadro's constant (designated NA) and thus: R = kBNA. Currently the most accurate value as published by the National Institute of Standards and Technology (NIST) is:[1] R = 8.3144621 J · K–1 · mol–1 A number of values of R in other units are provided in the adjacent table. The gas constant occurs in the ideal gas law as follows: PV = nRT or as: PVm = RT where: P is the absolute pressure of the gas T is the absolute temperature of the gas V is the volume the gas occupies n is the number of moles of gas Vm is the molar volume of the gas The specific gas constant The gas constant of a specific gas, as differentiated from the above universal molar gas constant which applies for any ideal gas, is designated by the symbol Rs and is equal to the molar gas constant divided by the molecular mass (M ) of the gas: Rs = R ÷ M The specific gas constant for an ideal gas may also be obtained from the following thermodynamicsrelationship:[2] Rs = cp – cv where cp and cv are the gas's specific heats at constant pressure and constant volume respectively. Some example values of the specific gas constant are: –1 –1 –1 Ammonia (molecular mass of 17.032 g · mol ) : Rs = 0.4882 J · K · g –1 –1 –1 Hydrogen (molecular mass of 2.016 g · mol ) : Rs = 4.1242 J · K · g –1 –1 –1 Methane (molecular mass of 16.043 g · mol ) : Rs = 0.5183 J · K · g Unfortunately, many authors in the technical literature sometimes use R as the specific gas constant without designating it as such or stating that it is the specific gas constant.