P = PRT Or Pv = RT
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Solutes and Solution
Solutes and Solution The first rule of solubility is “likes dissolve likes” Polar or ionic substances are soluble in polar solvents Non-polar substances are soluble in non- polar solvents Solutes and Solution There must be a reason why a substance is soluble in a solvent: either the solution process lowers the overall enthalpy of the system (Hrxn < 0) Or the solution process increases the overall entropy of the system (Srxn > 0) Entropy is a measure of the amount of disorder in a system—entropy must increase for any spontaneous change 1 Solutes and Solution The forces that drive the dissolution of a solute usually involve both enthalpy and entropy terms Hsoln < 0 for most species The creation of a solution takes a more ordered system (solid phase or pure liquid phase) and makes more disordered system (solute molecules are more randomly distributed throughout the solution) Saturation and Equilibrium If we have enough solute available, a solution can become saturated—the point when no more solute may be accepted into the solvent Saturation indicates an equilibrium between the pure solute and solvent and the solution solute + solvent solution KC 2 Saturation and Equilibrium solute + solvent solution KC The magnitude of KC indicates how soluble a solute is in that particular solvent If KC is large, the solute is very soluble If KC is small, the solute is only slightly soluble Saturation and Equilibrium Examples: + - NaCl(s) + H2O(l) Na (aq) + Cl (aq) KC = 37.3 A saturated solution of NaCl has a [Na+] = 6.11 M and [Cl-] = -

Thermodynamics Notes
Thermodynamics Notes Steven K. Krueger Department of Atmospheric Sciences, University of Utah August 2020 Contents 1 Introduction 1 1.1 What is thermodynamics? . .1 1.2 The atmosphere . .1 2 The Equation of State 1 2.1 State variables . .1 2.2 Charles' Law and absolute temperature . .2 2.3 Boyle's Law . .3 2.4 Equation of state of an ideal gas . .3 2.5 Mixtures of gases . .4 2.6 Ideal gas law: molecular viewpoint . .6 3 Conservation of Energy 8 3.1 Conservation of energy in mechanics . .8 3.2 Conservation of energy: A system of point masses . .8 3.3 Kinetic energy exchange in molecular collisions . .9 3.4 Working and Heating . .9 4 The Principles of Thermodynamics 11 4.1 Conservation of energy and the first law of thermodynamics . 11 4.1.1 Conservation of energy . 11 4.1.2 The first law of thermodynamics . 11 4.1.3 Work . 12 4.1.4 Energy transferred by heating . 13 4.2 Quantity of energy transferred by heating . 14 4.3 The first law of thermodynamics for an ideal gas . 15 4.4 Applications of the first law . 16 4.4.1 Isothermal process . 16 4.4.2 Isobaric process . 17 4.4.3 Isosteric process . 18 4.5 Adiabatic processes . 18 5 The Thermodynamics of Water Vapor and Moist Air 21 5.1 Thermal properties of water substance . 21 5.2 Equation of state of moist air . 21 5.3 Mixing ratio . 22 5.4 Moisture variables . 22 5.5 Changes of phase and latent heats . -
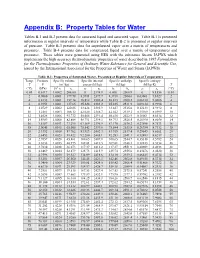
Appendix B: Property Tables for Water
Appendix B: Property Tables for Water Tables B-1 and B-2 present data for saturated liquid and saturated vapor. Table B-1 is presented information at regular intervals of temperature while Table B-2 is presented at regular intervals of pressure. Table B-3 presents data for superheated vapor over a matrix of temperatures and pressures. Table B-4 presents data for compressed liquid over a matrix of temperatures and pressures. These tables were generated using EES with the substance Steam_IAPWS which implements the high accuracy thermodynamic properties of water described in 1995 Formulation for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use, issued by the International Associated for the Properties of Water and Steam (IAPWS). Table B-1: Properties of Saturated Water, Presented at Regular Intervals of Temperature Temp. Pressure Specific volume Specific internal Specific enthalpy Specific entropy T P (m3/kg) energy (kJ/kg) (kJ/kg) (kJ/kg-K) T 3 (°C) (kPa) 10 vf vg uf ug hf hg sf sg (°C) 0.01 0.6117 1.0002 206.00 0 2374.9 0.000 2500.9 0 9.1556 0.01 2 0.7060 1.0001 179.78 8.3911 2377.7 8.3918 2504.6 0.03061 9.1027 2 4 0.8135 1.0001 157.14 16.812 2380.4 16.813 2508.2 0.06110 9.0506 4 6 0.9353 1.0001 137.65 25.224 2383.2 25.225 2511.9 0.09134 8.9994 6 8 1.0729 1.0002 120.85 33.626 2385.9 33.627 2515.6 0.12133 8.9492 8 10 1.2281 1.0003 106.32 42.020 2388.7 42.022 2519.2 0.15109 8.8999 10 12 1.4028 1.0006 93.732 50.408 2391.4 50.410 2522.9 0.18061 8.8514 12 14 1.5989 1.0008 82.804 58.791 2394.1 58.793 2526.5 -

Equation of State of an Ideal Gas
ASME231 Atmospheric Thermodynamics NC A&T State U Department of Physics Dr. Yuh-Lang Lin http://mesolab.org [email protected] Lecture 3: Equation of State of an Ideal Gas As mentioned earlier, the state of a system is the condition of the system (or part of the system) at an instant of time measured by its properties. Those properties are called state variables, such as T, p, and V. The equation which relates T, p, and V is called equation of state. Since they are related by the equation of state, only two of them are independent. Thus, all the other thermodynamic properties will depend on the state defined by two independent variables by state functions. PVT system: The simplest thermodynamic system consists of a fixed mass of a fluid uninfluenced by chemical reactions or external fields. Such a system can be described by the pressure, volume and temperature, which are related by an equation of state f (p, V, T) = 0, (2.1) 1 where only two of them are independent. From physical experiments, the equation of state for an ideal gas, which is defined as a hypothetical gas whose molecules occupy negligible space and have no interactions, may be written as p = RT, (2.2) or p = RT, (2.3) where -3 : density (=m/V) in kg m , 3 -1 : specific volume (=V/m=1/) in m kg . R: specific gas constant (R=R*/M), -1 -1 -1 -1 [R=287 J kg K for dry air (Rd), 461 J kg K for water vapor for water vapor (Rv)]. -

Air Pressure
Name ____________________________________ Date __________ Class ___________________ SECTION 15-3 SECTION SUMMARY Air Pressure Guide for ir consists of atoms and molecules that have mass. Therefore, air has Reading A mass. Because air has mass, it also has other properties, includ- ing density and pressure. The amount of mass per unit volume of a N What are some of substance is called the density of the substance. The force per unit area the properties of air? is called pressure. Air pressure is the result of the weight of a column of N What instruments air pushing down on an area. The molecules in air push in all directions. are used to mea- This is why air pressure doesn’t crush objects. sure air pressure? Falling air pressure usually indicates that a storm is approaching. Rising N How does increas- air pressure usually means that the weather is clearing. A ing altitude affect barometer is an instrument that measures changes in air pressure. There air pressure and are two kinds of barometers: mercury barometers and aneroid density? barometers. A mercury barometer consists of a glass tube open at the bottom end and partially filled with mercury. The open end of the tube rests in a dish of mercury, and the space above the mercury in the tube contains no air. The air pressure pushing down on the surface of the mer- cury in the dish is equal to the weight of the column of mercury in the tube. At sea level, the mercury column is about 76 centimeters high, on average. An aneroid barometer has an airtight metal chamber that is sen- sitive to changes in air pressure. -

Effect of Tropospheric Air Density and Dew Point Temperature on Radio (Electromagnetic) Waves and Air Radio Wave Refractivity
International Journal of Scientific & Engineering Research, Volume 7, Issue 6, June-2016 356 ISSN 2229-5518 Effect of Tropospheric Air density and dew point temperature on Radio (Electromagnetic) waves and Air radio wave refractivity <Joseph Amajama> University of Calabar, Department of Physics, Electronics and Computer Technology Unit Etta-agbor, Calabar, Nigeria [email protected] Abstract: Signal strengths measurements were obtained half hourly for some hours and simultaneously, the atmospheric components: atmospheric temperature, atmospheric pressure, relative humidity and wind direction and speed were registered to erect the effects of air density and dew point temperature on radio signals (electromagnetic waves) as they travel through the atmosphere and air radio wave refractivity. The signal strength from Cross River State Broadcasting Co-operation Television (CRBC-TV), (4057'54.7''N, 8019'43.7''E) transmitted at 35mdB and 519.25 MHz (UHF) were measured using a Cable TV analyzer in a residence along Ettaabgor, Calabar, Nigeria (4057'31.7''N, 8020'49.7''E) using the digital Community – Access (Cable) Television (CATV) analyzer with 24 channels, spectrum 46 – 870 MHz, connected to a domestic receiver antenna of height 4.23 m. Results show that: on the condition that the wind speed and direction are the same or (0 mph NA), the radio signal strength is near negligibly directly proportional to the air density, mathematically Ss / ∂a1.3029 = K, where Ss is Signal Strength in dB, ∂a is Density of air Kg/m3 and K is constant; radio -

Air Infiltration Glossary (English Edition)
AIRGLOSS: Air Infiltration Glossary (English Edition) Carolyn Allen ~)Copyrlght Oscar Faber Partnership 1981. All property rights, Including copyright ere vested In the Operating Agent (The Oscar Faber Partnership) on behalf of the International Energy Agency. In particular, no part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior written permluion of the operat- ing agent. Contents (i) Preface (iii) Introduction (v) Umr's Guide (v) Glossary Appendix 1 - References 87 Appendix 2 - Tracer Gases 93 Appendix 3 - Abbreviations 99 Appendix 4 - Units 103 (i) (il) Preface International Energy Agency In order to strengthen cooperation In the vital area of energy policy, an Agreement on an International Energy Program was formulated among a number of industrialised countries In November 1974. The International Energy Agency (lEA) was established as an autonomous body within the Organisation for Economic Cooperation and Development (OECD) to administer that agreement. Twenty-one countries are currently members of the lEA, with the Commission of the European Communities participating under a special arrangement. As one element of the International Energy Program, the Participants undertake cooperative activities in energy research, development, and demonstration. A number of new and improved energy technologies which have the potential of making significant contributions to our energy needs were identified for collaborative efforts. The lEA Committee on Energy Research and Development (CRD), assisted by a small Secretariat staff, coordinates the energy research, development, and demonstration programme. Energy Conservation in Buildings and Community Systems The International Energy Agency sponsors research and development in a number of areas related to energy. -

Calculation Exercises with Answers and Solutions
Atmospheric Chemistry and Physics Calculation Exercises Contents Exercise A, chapter 1 - 3 in Jacob …………………………………………………… 2 Exercise B, chapter 4, 6 in Jacob …………………………………………………… 6 Exercise C, chapter 7, 8 in Jacob, OH on aerosols and booklet by Heintzenberg … 11 Exercise D, chapter 9 in Jacob………………………………………………………. 16 Exercise E, chapter 10 in Jacob……………………………………………………… 20 Exercise F, chapter 11 - 13 in Jacob………………………………………………… 24 Answers and solutions …………………………………………………………………. 29 Note that approximately 40% of the written exam deals with calculations. The remainder is about understanding of the theory. Exercises marked with an asterisk (*) are for the most interested students. These exercises are more comprehensive and/or difficult than questions appearing in the written exam. 1 Atmospheric Chemistry and Physics – Exercise A, chap. 1 – 3 Recommended activity before exercise: Try to solve 1:1 – 1:5, 2:1 – 2:2 and 3:1 – 3:2. Summary: Concentration Example Advantage Number density No. molecules/m3, Useful for calculations of reaction kmol/m3 rates in the gas phase Partial pressure Useful measure on the amount of a substance that easily can be converted to mixing ratio Mixing ratio ppmv can mean e.g. Concentration relative to the mole/mole or partial concentration of air molecules. Very pressure/total pressure useful because air is compressible. Ideal gas law: PV = nRT Molar mass: M = m/n Density: ρ = m/V = PM/RT; (from the two equations above) Mixing ratio (vol): Cx = nx/na = Px/Pa ≠ mx/ma Number density: Cvol = nNav/V 26 -1 Avogadro’s -

CE-087 Calculation of Gas Density and Viscosity
CE‐087 Calculation of Gas Density and Viscosity Instructor: Harlan Bengtson, PhD, P.E. Course ID: CE‐087 PDH Hours: 2 PDH PDH Star | T / F: (833) PDH‐STAR (734‐7827) | E: [email protected] Calculation of Gas Density and Viscosity Harlan H. Bengtson, PhD, P.E. COURSE CONTENT 1. Introduction The density and/or viscosity of a gas is often needed for some other calculation, such as pipe flow or heat exchanger calculations. This course contains discussion of, and example calculation of, the density and viscosity of a specified gas at a given temperature and pressure. If the gas temperature is high relative to its critical temperature and the gas pressure is low relative to its critical pressure, then it can be treated as an ideal gas and its density can be calculated at a specified temperature and pressure using the ideal gas law. If the density of a gas is needed at a temperature and pressure at which it cannot be treated as an ideal gas law, however, then the compressibility factor of the gas must be calculated and used in calculating its density. In this course, the Redlich Kwong equation will be used for calculation of the compressibility factor of a gas. The Sutherland formula can be used to calculate the viscosity of a gas at a specified temperature and pressure if the Sutherland constants are available for the gas. It will be discussed and used in example calculations. Another method for calculating the viscosity of air at a specified temperature and pressure will also be presented and discussed. -
![[5] Magnetic Densimetry: Partial Specific Volume and Other Applications](https://docslib.b-cdn.net/cover/6742/5-magnetic-densimetry-partial-specific-volume-and-other-applications-566742.webp)
[5] Magnetic Densimetry: Partial Specific Volume and Other Applications
74 MOLECULAR WEIGHT DETERMINATIONS [5] [5] Magnetic Densimetry: Partial Specific Volume and Other Applications By D. W. KUPKE and J. W. BEAMS This chapter consists of three parts. Part I is a summary of the principles and current development of the magnetic densimeter, with which density values may be obtained conveniently on small volumes of solution with the speed and accuracy required for present-day ap- plications in protein chemistry. In Part II, the applications, including definitions, are outlined whereby the density property may be utilized for the study of protein solutions. Finally, in Part III, some practical aspects on the routine determination of densities of protein solutions by magnetic densimetry are listed. Part I. Magnetic Densimeter The magnetic densimeter measures by electromagnetic methods the vertical (up or down) force on a totally immersed buoy. From these measured forces together with the known mass and volume of the buoy, the density of the solution can be obtained by employing Archimedes' principle. In practice, it is convenient to eliminate evaluation of the mass and volume of the buoy and to determine the relation of the mag- netic to the mechanical forces on the buoy by direct calibration of the latter when it is immersed in liquids of known density. Several workers have devised magnetic float methods for determin- ing densities, but the method first described by Lamb and Lee I and later improved by MacInnes, Dayhoff, and Ray: is perhaps the most accurate means (~1 part in 106) devised up to that time for determining the densities of solutions. The magnetic method was not widely used, first because it was tedious and required considerable manipulative skill; second, the buoy or float was never stationary for periods long enough to allow ruling out of wall effects, viscosity perturbations, etc.; third, the technique required comparatively large volumes of the solution (350 ml) for accurate measurements. -

Ideal Gas Law PV = Nrt R = Universal Gas Constant R = 0.08206 L-Atm R = 8.314 J Mol-K Mol-K Example
Ideal gas law PV = nRT R = universal gas constant R = 0.08206 L-atm R = 8.314 J mol-K mol-K Example: In the reaction of oxygen with carbon to produce carbon dioxide, how many liters of oxygen at 747 mm Hg and 21.0 °C are needed to react with 24.022 g of carbon? CHEM 102 Winter 2011 Practice: In the reaction of oxygen and hydrogen to make water, how many liters of hydrogen at exactly 0 °C and 1 atm are needed to react with 10.0 g of oxygen? 2 CHEM 102 Winter 2011 Combined gas law V decreases with pressure (V α 1/P). V increases with temperature (V α T). V increases with particles (V α n). Combining these ideas: V α n x T P For a given amount of gas (constant number of particles, ninitial = nafter) that changes in volume, temperature, or pressure: Vinitial α ninitial x Tinitial Pinitial Vafter α nafter x Tafter Pafter ninitial = n after Vinitial x Pinitial = Vafter x Pafter Tinitial Tafter 3 CHEM 102 Winter 2011 Example: A sample of gas is pressurized to 1.80 atm and volume of 90.0 cm3. What volume would the gas have if it were pressurized to 4.00 atm without a change in temperature? 4 CHEM 102 Winter 2011 Example: A 2547-mL sample of gas is at 421 °C. What is the new gas temperature if the gas is cooled at constant pressure until it has a volume of 1616 mL? 5 CHEM 102 Winter 2011 Practice: A balloon is filled with helium. -

Chapter 3 3.4-2 the Compressibility Factor Equation of State
Chapter 3 3.4-2 The Compressibility Factor Equation of State The dimensionless compressibility factor, Z, for a gaseous species is defined as the ratio pv Z = (3.4-1) RT If the gas behaves ideally Z = 1. The extent to which Z differs from 1 is a measure of the extent to which the gas is behaving nonideally. The compressibility can be determined from experimental data where Z is plotted versus a dimensionless reduced pressure pR and reduced temperature TR, defined as pR = p/pc and TR = T/Tc In these expressions, pc and Tc denote the critical pressure and temperature, respectively. A generalized compressibility chart of the form Z = f(pR, TR) is shown in Figure 3.4-1 for 10 different gases. The solid lines represent the best curves fitted to the data. Figure 3.4-1 Generalized compressibility chart for various gases10. It can be seen from Figure 3.4-1 that the value of Z tends to unity for all temperatures as pressure approach zero and Z also approaches unity for all pressure at very high temperature. If the p, v, and T data are available in table format or computer software then you should not use the generalized compressibility chart to evaluate p, v, and T since using Z is just another approximation to the real data. 10 Moran, M. J. and Shapiro H. N., Fundamentals of Engineering Thermodynamics, Wiley, 2008, pg. 112 3-19 Example 3.4-2 ---------------------------------------------------------------------------------- A closed, rigid tank filled with water vapor, initially at 20 MPa, 520oC, is cooled until its temperature reaches 400oC.