Session 15 Thermodynamics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thermodynamics Notes
Thermodynamics Notes Steven K. Krueger Department of Atmospheric Sciences, University of Utah August 2020 Contents 1 Introduction 1 1.1 What is thermodynamics? . .1 1.2 The atmosphere . .1 2 The Equation of State 1 2.1 State variables . .1 2.2 Charles' Law and absolute temperature . .2 2.3 Boyle's Law . .3 2.4 Equation of state of an ideal gas . .3 2.5 Mixtures of gases . .4 2.6 Ideal gas law: molecular viewpoint . .6 3 Conservation of Energy 8 3.1 Conservation of energy in mechanics . .8 3.2 Conservation of energy: A system of point masses . .8 3.3 Kinetic energy exchange in molecular collisions . .9 3.4 Working and Heating . .9 4 The Principles of Thermodynamics 11 4.1 Conservation of energy and the first law of thermodynamics . 11 4.1.1 Conservation of energy . 11 4.1.2 The first law of thermodynamics . 11 4.1.3 Work . 12 4.1.4 Energy transferred by heating . 13 4.2 Quantity of energy transferred by heating . 14 4.3 The first law of thermodynamics for an ideal gas . 15 4.4 Applications of the first law . 16 4.4.1 Isothermal process . 16 4.4.2 Isobaric process . 17 4.4.3 Isosteric process . 18 4.5 Adiabatic processes . 18 5 The Thermodynamics of Water Vapor and Moist Air 21 5.1 Thermal properties of water substance . 21 5.2 Equation of state of moist air . 21 5.3 Mixing ratio . 22 5.4 Moisture variables . 22 5.5 Changes of phase and latent heats . -
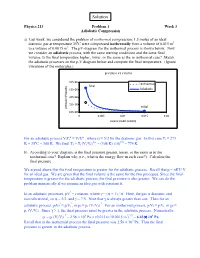
3-1 Adiabatic Compression
Solution Physics 213 Problem 1 Week 3 Adiabatic Compression a) Last week, we considered the problem of isothermal compression: 1.5 moles of an ideal diatomic gas at temperature 35oC were compressed isothermally from a volume of 0.015 m3 to a volume of 0.0015 m3. The pV-diagram for the isothermal process is shown below. Now we consider an adiabatic process, with the same starting conditions and the same final volume. Is the final temperature higher, lower, or the same as the in isothermal case? Sketch the adiabatic processes on the p-V diagram below and compute the final temperature. (Ignore vibrations of the molecules.) α α For an adiabatic process ViTi = VfTf , where α = 5/2 for the diatomic gas. In this case Ti = 273 o 1/α 2/5 K + 35 C = 308 K. We find Tf = Ti (Vi/Vf) = (308 K) (10) = 774 K. b) According to your diagram, is the final pressure greater, lesser, or the same as in the isothermal case? Explain why (i.e., what is the energy flow in each case?). Calculate the final pressure. We argued above that the final temperature is greater for the adiabatic process. Recall that p = nRT/ V for an ideal gas. We are given that the final volume is the same for the two processes. Since the final temperature is greater for the adiabatic process, the final pressure is also greater. We can do the problem numerically if we assume an idea gas with constant α. γ In an adiabatic processes, pV = constant, where γ = (α + 1) / α. -

Thermodynamics Formulation of Economics Burin Gumjudpai A
Thermodynamics Formulation of Economics 306706052 NIDA E-THESIS 5710313001 thesis / recv: 09012563 15:07:41 seq: 16 Burin Gumjudpai A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Economics (Financial Economics) School of Development Economics National Institute of Development Administration 2019 Thermodynamics Formulation of Economics Burin Gumjudpai School of Development Economics Major Advisor (Associate Professor Yuthana Sethapramote, Ph.D.) The Examining Committee Approved This Thesis Submitted in Partial 306706052 Fulfillment of the Requirements for the Degree of Master of Economics (Financial Economics). Committee Chairperson NIDA E-THESIS 5710313001 thesis / recv: 09012563 15:07:41 seq: 16 (Assistant Professor Pongsak Luangaram, Ph.D.) Committee (Assistant Professor Athakrit Thepmongkol, Ph.D.) Committee (Associate Professor Yuthana Sethapramote, Ph.D.) Dean (Associate Professor Amornrat Apinunmahakul, Ph.D.) ______/______/______ ABST RACT ABSTRACT Title of Thesis Thermodynamics Formulation of Economics Author Burin Gumjudpai Degree Master of Economics (Financial Economics) Year 2019 306706052 We consider a group of information-symmetric consumers with one type of commodity in an efficient market. The commodity is fixed asset and is non-disposable. We are interested in seeing if there is some connection of thermodynamics formulation NIDA E-THESIS 5710313001 thesis / recv: 09012563 15:07:41 seq: 16 to microeconomics. We follow Carathéodory approach which requires empirical existence of equation of state (EoS) and coordinates before performing maximization of variables. In investigating EoS of various system for constructing the economics EoS, unexpectedly new insights of thermodynamics are discovered. With definition of truly endogenous function, criteria rules and diagrams are proposed in identifying the status of EoS for an empirical equation. -

Thermodynamics
ME346A Introduction to Statistical Mechanics { Wei Cai { Stanford University { Win 2011 Handout 6. Thermodynamics January 26, 2011 Contents 1 Laws of thermodynamics 2 1.1 The zeroth law . .3 1.2 The first law . .4 1.3 The second law . .5 1.3.1 Efficiency of Carnot engine . .5 1.3.2 Alternative statements of the second law . .7 1.4 The third law . .8 2 Mathematics of thermodynamics 9 2.1 Equation of state . .9 2.2 Gibbs-Duhem relation . 11 2.2.1 Homogeneous function . 11 2.2.2 Virial theorem / Euler theorem . 12 2.3 Maxwell relations . 13 2.4 Legendre transform . 15 2.5 Thermodynamic potentials . 16 3 Worked examples 21 3.1 Thermodynamic potentials and Maxwell's relation . 21 3.2 Properties of ideal gas . 24 3.3 Gas expansion . 28 4 Irreversible processes 32 4.1 Entropy and irreversibility . 32 4.2 Variational statement of second law . 32 1 In the 1st lecture, we will discuss the concepts of thermodynamics, namely its 4 laws. The most important concepts are the second law and the notion of Entropy. (reading assignment: Reif x 3.10, 3.11) In the 2nd lecture, We will discuss the mathematics of thermodynamics, i.e. the machinery to make quantitative predictions. We will deal with partial derivatives and Legendre transforms. (reading assignment: Reif x 4.1-4.7, 5.1-5.12) 1 Laws of thermodynamics Thermodynamics is a branch of science connected with the nature of heat and its conver- sion to mechanical, electrical and chemical energy. (The Webster pocket dictionary defines, Thermodynamics: physics of heat.) Historically, it grew out of efforts to construct more efficient heat engines | devices for ex- tracting useful work from expanding hot gases (http://www.answers.com/thermodynamics). -

1 / C\% Cellular Motions and Thermal Fluctuations: the Brownian Ratchet
1 / C\% 316 Biophysical Journal Volume 65 July 1993 316-324 ” Cellular Motions and Thermal Fluctuations: The Brownian Ratchet Charles S. Peskin,* Garrett M. Odell,* and George F. Osters l Courant Institute of Mathematical Sciences, New York, New York 10012; *Department of Zoology, University of Washington, Seattle, Washington 98195; SDepartments of Molecular and Cellular Biology, and Entomology, University of California, Berkeley, California 94720 USA ABSTRACT We present here a model for how chemical reactions generate protrusive forces by rectifying Brownian motion. This sort of energy transduction drives a number of intracellular processes, including filopodial protrusion, propulsion of the bacterium Wisteria, and protein translocation. INTRODUCTION Many types of cellular protrusions, including filopodia, This is the speed of a perfect BR. Note that as the ratchet lamellipodia, and acrosomal extension do not appear to in- interval, 8, decreases, the ratchet velocity increases. This is volve molecular motors. These processes transduce chemical because the frequency of smaller Brownian steps grows more bond energy into directed motion, but they do not operate in rapidly than the step size shrinks (when 6 is of the order of a mechanochemical cycle and need not depend directly upon a mean free path, then this formula obviously breaks down). nucleotide hydrolysis. In this paper we describe several such Several ingredients must be added to this simple expres- processes and present simple formulas for the velocity and sion to make it useful in real situations. First, the ratchet force they generate. We shall call these machines “Brownian cannot be perfect: a particle crossing a ratchet boundary may ratchets” (BR) because rectified Brownian motion is funda- occasionally cross back. -

Chapter03 Introduction to Thermodynamic Systems
Chapter03 Introduction to Thermodynamic Systems February 13, 2019 This chapter introduces the concept of thermo- Remark 1.1. In general, we will consider three kinds dynamic systems and digresses on the issue: How of exchange between S and Se through a (possibly ab- complex systems are related to Statistical Thermo- stract) membrane: dynamics. To do so, let us review the concepts of (1) Diathermal (Contrary: Adiabatic) membrane Equilibrium Thermodynamics from the conventional that allows exchange of thermal energy. viewpoint. Let U be the universe. Let a collection of objects (2) Deformable (Contrary: Rigid) membrane that S ⊆ U be defined as the system under certain restric- allows exchange of work done by volume forces tive conditions. The complement of S in U is called or surface forces. the exterior of S and is denoted as Se. (3) Permeable (Contrary: Impermeable) membrane that allows exchange of matter (e.g., in the form 1 Basic Definitions of molecules of chemical species). Definition 1.1. A system S is an idealized body that can be isolated from Se in the sense that changes in Se Definition 1.4. A thermodynamic state variable is a do not have any effects on S. macroscopic entity that is a characteristic of a system S. A thermodynamic state variable can be a scalar, Definition 1.2. A system S is called a thermody- a finite-dimensional vector, or a tensor. Examples: namic system if any possible exchange between S and temperature, or a strain tensor. Se is restricted to one or more of the following: (1) Any form of energy (e.g., thermal, electric, and magnetic) Definition 1.5. -

On Control of Transport in Brownian Ratchet Mechanisms
Journal of Process Control 27 (2015) 76–86 Contents lists available at ScienceDirect Journal of Process Control j ournal homepage: www.elsevier.com/locate/jprocont On control of transport in Brownian ratchet mechanisms a,∗ a b a Subhrajit Roychowdhury , Govind Saraswat , Srinivasa Salapaka , Murti Salapaka a Department of Electrical and Computer Engineering, University of Minnesota-Twin Cities, 2-270 Keller Hall, Minneapolis, MN 55455, USA b Department of Mechanical Science and Engineering, University of Illinois at Urbana-Champaign, 1206 West Green Street, Urbana, IL 61801, USA a r t i c l e i n f o a b s t r a c t Article history: Engineered transport of material at the nano/micro scale is essential for the manufacturing platforms of Received 1 April 2014 the future. Unlike conventional transport systems, at the nano/micro scale, transport has to be achieved Received in revised form in the presence of fundamental sources of uncertainty such as thermal noise. Remarkably, it is possible 15 September 2014 to extract useful work by rectifying noise using an asymmetric potential; a principle used by Brownian Accepted 26 November 2014 ratchets. In this article a systematic methodology for designing open-loop Brownian ratchet mechanisms Available online 23 December 2014 that optimize velocity and efficiency is developed. In the case where the particle position is available as a measured variable, closed loop methodologies are studied. Here, it is shown that methods that strive Keywords: to optimize velocity of transport may compromise efficiency. A dynamic programming based approach Flashing ratchet is presented which yields up to three times improvement in efficiency over optimized open loop designs Stochastic systems Dynamic programming and 35% better efficiency over reported closed loop strategies that focus on optimizing velocities. -

Statistical Thermodynamics - Fall 2009
1 Statistical Thermodynamics - Fall 2009 Professor Dmitry Garanin Thermodynamics September 9, 2012 I. PREFACE The course of Statistical Thermodynamics consist of two parts: Thermodynamics and Statistical Physics. These both branches of physics deal with systems of a large number of particles (atoms, molecules, etc.) at equilibrium. 3 19 One cm of an ideal gas under normal conditions contains NL =2.69 10 atoms, the so-called Loschmidt number. Although one may describe the motion of the atoms with the help of× Newton’s equations, direct solution of such a large number of differential equations is impossible. On the other hand, one does not need the too detailed information about the motion of the individual particles, the microscopic behavior of the system. One is rather interested in the macroscopic quantities, such as the pressure P . Pressure in gases is due to the bombardment of the walls of the container by the flying atoms of the contained gas. It does not exist if there are only a few gas molecules. Macroscopic quantities such as pressure arise only in systems of a large number of particles. Both thermodynamics and statistical physics study macroscopic quantities and relations between them. Some macroscopics quantities, such as temperature and entropy, are non-mechanical. Equilibruim, or thermodynamic equilibrium, is the state of the system that is achieved after some time after time-dependent forces acting on the system have been switched off. One can say that the system approaches the equilibrium, if undisturbed. Again, thermodynamic equilibrium arises solely in macroscopic systems. There is no thermodynamic equilibrium in a system of a few particles that are moving according to the Newton’s law. -

Lecture 6: Entropy
Matthew Schwartz Statistical Mechanics, Spring 2019 Lecture 6: Entropy 1 Introduction In this lecture, we discuss many ways to think about entropy. The most important and most famous property of entropy is that it never decreases Stot > 0 (1) Here, Stot means the change in entropy of a system plus the change in entropy of the surroundings. This is the second law of thermodynamics that we met in the previous lecture. There's a great quote from Sir Arthur Eddington from 1927 summarizing the importance of the second law: If someone points out to you that your pet theory of the universe is in disagreement with Maxwell's equationsthen so much the worse for Maxwell's equations. If it is found to be contradicted by observationwell these experimentalists do bungle things sometimes. But if your theory is found to be against the second law of ther- modynamics I can give you no hope; there is nothing for it but to collapse in deepest humiliation. Another possibly relevant quote, from the introduction to the statistical mechanics book by David Goodstein: Ludwig Boltzmann who spent much of his life studying statistical mechanics, died in 1906, by his own hand. Paul Ehrenfest, carrying on the work, died similarly in 1933. Now it is our turn to study statistical mechanics. There are many ways to dene entropy. All of them are equivalent, although it can be hard to see. In this lecture we will compare and contrast dierent denitions, building up intuition for how to think about entropy in dierent contexts. The original denition of entropy, due to Clausius, was thermodynamic. -

Brownian Ratchets in Physics and Biology
Contemporary Physics, 1997, volume 38, number 6, pages 371 ± 379 Brownian ratchets in physics and biology M ARTIN BIER Thirty years ago Feynman et al. presented a paradox in the Lectures on Physics: an imagined device could let Brownian motion do work by allowing it in one direction and blocking it in the opposite direction. In the chapter Feynman et al. eventually show that such ratcheting can only be achieved if there is, in compliance with the basic conservation laws, some energy input from an external source. Now that technology is going into ever smaller dimensions, ratcheting Brownian motion seems to be a real possibility in nanotechnological applications. Furthermore, Brownian motion plays an essential role in the action of motor proteins (individual molecules that convert chemical energy into motion). 1. The thermodynamic consistency of a Brownian microscopic engine is not necessarily the microscopic ratchet equivalent of an e cient macroscopic engine. At the Technology is reaching into ever smaller dimensions microscopic level it is possible to `ratchet’ Brownian nowadays. Many research groups are shrinking labs onto motion, i.e. to allow Brownian motion in one direction tiny squares of silicon or glass. On these `labs on chips’ and block it in the opposite direction so net displacement individual bacteria viruses and macromolecules (like occurs. It appears that a lot of biological systems at the proteins or DNA strands) are identi® ed and/or manipu- molecular level operate like this [3]. Systems that convert lated [1]. On the micrometre scale physics is diŒerent. For energy in the presence of Brownian motion are compli- motion in a ¯ uid the Reynolds number (i.e. -

The First Law of Thermodynamics Continued Pre-Reading: §19.5 Where We Are
Lecture 7 The first law of thermodynamics continued Pre-reading: §19.5 Where we are The pressure p, volume V, and temperature T are related by an equation of state. For an ideal gas, pV = nRT = NkT For an ideal gas, the temperature T is is a direct measure of the average kinetic energy of its 3 3 molecules: KE = nRT = NkT tr 2 2 2 3kT 3RT and vrms = (v )av = = r m r M p Where we are We define the internal energy of a system: UKEPE=+∑∑ interaction Random chaotic between atoms motion & molecules For an ideal gas, f UNkT= 2 i.e. the internal energy depends only on its temperature Where we are By considering adding heat to a fixed volume of an ideal gas, we showed f f Q = Nk∆T = nR∆T 2 2 and so, from the definition of heat capacity Q = nC∆T f we have that C = R for any ideal gas. V 2 Change in internal energy: ∆U = nCV ∆T Heat capacity of an ideal gas Now consider adding heat to an ideal gas at constant pressure. By definition, Q = nCp∆T and W = p∆V = nR∆T So from ∆U = Q W − we get nCV ∆T = nCp∆T nR∆T − or Cp = CV + R It takes greater heat input to raise the temperature of a gas a given amount at constant pressure than constant volume YF §19.4 Ratio of heat capacities Look at the ratio of these heat capacities: we have f C = R V 2 and f + 2 C = C + R = R p V 2 so C p γ = > 1 CV 3 For a monatomic gas, CV = R 3 5 2 so Cp = R + R = R 2 2 C 5 R 5 and γ = p = 2 = =1.67 C 3 R 3 YF §19.4 V 2 Problem An ideal gas is enclosed in a cylinder which has a movable piston. -

Adiabatic Bulk Moduli
8.03 at ESG Supplemental Notes Adiabatic Bulk Moduli To find the speed of sound in a gas, or any property of a gas involving elasticity (see the discussion of the Helmholtz oscillator, B&B page 22, or B&B problem 1.6, or French Pages 57-59.), we need the “bulk modulus” of the fluid. This will correspond to the “spring constant” of a spring, and will give the magnitude of the restoring agency (pressure for a gas, force for a spring) in terms of the change in physical dimension (volume for a gas, length for a spring). It turns out to be more useful to use an intensive quantity for the bulk modulus of a gas, so what we want is the change in pressure per fractional change in volume, so the bulk modulus, denoted as κ (the Greek “kappa” which, when written, has a great tendency to look like k, and in fact French uses “K”), is ∆p dp κ = − , or κ = −V . ∆V/V dV The minus sign indicates that for normal fluids (not all are!), a negative change in volume results in an increase in pressure. To find the bulk modulus, we need to know something about the gas and how it behaves. For our purposes, we will need three basic principles (actually 2 1/2) which we get from thermodynamics, or the kinetic theory of gasses. You might well have encountered these in previous classes, such as chemistry. A) The ideal gas law; pV = nRT , with the standard terminology. B) The first law of thermodynamics; dU = dQ − pdV,whereUis internal energy and Q is heat.