Heat Capacity Ratio of Gases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
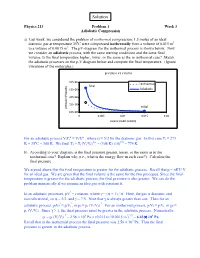
3-1 Adiabatic Compression
Solution Physics 213 Problem 1 Week 3 Adiabatic Compression a) Last week, we considered the problem of isothermal compression: 1.5 moles of an ideal diatomic gas at temperature 35oC were compressed isothermally from a volume of 0.015 m3 to a volume of 0.0015 m3. The pV-diagram for the isothermal process is shown below. Now we consider an adiabatic process, with the same starting conditions and the same final volume. Is the final temperature higher, lower, or the same as the in isothermal case? Sketch the adiabatic processes on the p-V diagram below and compute the final temperature. (Ignore vibrations of the molecules.) α α For an adiabatic process ViTi = VfTf , where α = 5/2 for the diatomic gas. In this case Ti = 273 o 1/α 2/5 K + 35 C = 308 K. We find Tf = Ti (Vi/Vf) = (308 K) (10) = 774 K. b) According to your diagram, is the final pressure greater, lesser, or the same as in the isothermal case? Explain why (i.e., what is the energy flow in each case?). Calculate the final pressure. We argued above that the final temperature is greater for the adiabatic process. Recall that p = nRT/ V for an ideal gas. We are given that the final volume is the same for the two processes. Since the final temperature is greater for the adiabatic process, the final pressure is also greater. We can do the problem numerically if we assume an idea gas with constant α. γ In an adiabatic processes, pV = constant, where γ = (α + 1) / α. -

Thermodynamics Formulation of Economics Burin Gumjudpai A
Thermodynamics Formulation of Economics 306706052 NIDA E-THESIS 5710313001 thesis / recv: 09012563 15:07:41 seq: 16 Burin Gumjudpai A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Economics (Financial Economics) School of Development Economics National Institute of Development Administration 2019 Thermodynamics Formulation of Economics Burin Gumjudpai School of Development Economics Major Advisor (Associate Professor Yuthana Sethapramote, Ph.D.) The Examining Committee Approved This Thesis Submitted in Partial 306706052 Fulfillment of the Requirements for the Degree of Master of Economics (Financial Economics). Committee Chairperson NIDA E-THESIS 5710313001 thesis / recv: 09012563 15:07:41 seq: 16 (Assistant Professor Pongsak Luangaram, Ph.D.) Committee (Assistant Professor Athakrit Thepmongkol, Ph.D.) Committee (Associate Professor Yuthana Sethapramote, Ph.D.) Dean (Associate Professor Amornrat Apinunmahakul, Ph.D.) ______/______/______ ABST RACT ABSTRACT Title of Thesis Thermodynamics Formulation of Economics Author Burin Gumjudpai Degree Master of Economics (Financial Economics) Year 2019 306706052 We consider a group of information-symmetric consumers with one type of commodity in an efficient market. The commodity is fixed asset and is non-disposable. We are interested in seeing if there is some connection of thermodynamics formulation NIDA E-THESIS 5710313001 thesis / recv: 09012563 15:07:41 seq: 16 to microeconomics. We follow Carathéodory approach which requires empirical existence of equation of state (EoS) and coordinates before performing maximization of variables. In investigating EoS of various system for constructing the economics EoS, unexpectedly new insights of thermodynamics are discovered. With definition of truly endogenous function, criteria rules and diagrams are proposed in identifying the status of EoS for an empirical equation. -

Isobaric Expansion Engines: New Opportunities in Energy Conversion for Heat Engines, Pumps and Compressors
energies Concept Paper Isobaric Expansion Engines: New Opportunities in Energy Conversion for Heat Engines, Pumps and Compressors Maxim Glushenkov 1, Alexander Kronberg 1,*, Torben Knoke 2 and Eugeny Y. Kenig 2,3 1 Encontech B.V. ET/TE, P.O. Box 217, 7500 AE Enschede, The Netherlands; [email protected] 2 Chair of Fluid Process Engineering, Paderborn University, Pohlweg 55, 33098 Paderborn, Germany; [email protected] (T.K.); [email protected] (E.Y.K.) 3 Chair of Thermodynamics and Heat Engines, Gubkin Russian State University of Oil and Gas, Leninsky Prospekt 65, Moscow 119991, Russia * Correspondence: [email protected] or [email protected]; Tel.: +31-53-489-1088 Received: 12 December 2017; Accepted: 4 January 2018; Published: 8 January 2018 Abstract: Isobaric expansion (IE) engines are a very uncommon type of heat-to-mechanical-power converters, radically different from all well-known heat engines. Useful work is extracted during an isobaric expansion process, i.e., without a polytropic gas/vapour expansion accompanied by a pressure decrease typical of state-of-the-art piston engines, turbines, etc. This distinctive feature permits isobaric expansion machines to serve as very simple and inexpensive heat-driven pumps and compressors as well as heat-to-shaft-power converters with desired speed/torque. Commercial application of such machines, however, is scarce, mainly due to a low efficiency. This article aims to revive the long-known concept by proposing important modifications to make IE machines competitive and cost-effective alternatives to state-of-the-art heat conversion technologies. Experimental and theoretical results supporting the isobaric expansion technology are presented and promising potential applications, including emerging power generation methods, are discussed. -

Section 15-6: Thermodynamic Cycles
Answer to Essential Question 15.5: The ideal gas law tells us that temperature is proportional to PV. for state 2 in both processes we are considering, so the temperature in state 2 is the same in both cases. , and all three factors on the right-hand side are the same for the two processes, so the change in internal energy is the same (+360 J, in fact). Because the gas does no work in the isochoric process, and a positive amount of work in the isobaric process, the First Law tells us that more heat is required for the isobaric process (+600 J versus +360 J). 15-6 Thermodynamic Cycles Many devices, such as car engines and refrigerators, involve taking a thermodynamic system through a series of processes before returning the system to its initial state. Such a cycle allows the system to do work (e.g., to move a car) or to have work done on it so the system can do something useful (e.g., removing heat from a fridge). Let’s investigate this idea. EXPLORATION 15.6 – Investigate a thermodynamic cycle One cycle of a monatomic ideal gas system is represented by the series of four processes in Figure 15.15. The process taking the system from state 4 to state 1 is an isothermal compression at a temperature of 400 K. Complete Table 15.1 to find Q, W, and for each process, and for the entire cycle. Process Special process? Q (J) W (J) (J) 1 ! 2 No +1360 2 ! 3 Isobaric 3 ! 4 Isochoric 0 4 ! 1 Isothermal 0 Entire Cycle No 0 Table 15.1: Table to be filled in to analyze the cycle. -

Thermodynamics I - Enthalpy
CHEM 2880 - Kinetics Thermodynamics I - Enthalpy Tinoco Chapter 2 Secondary Reference: J.B. Fenn, Engines, Energy and Entropy, Global View Publishing, Pittsburgh, 2003. 1 CHEM 2880 - Kinetics Thermodynamics • An essential foundation for understanding physical and biological sciences. • Relationships and interconversions between various forms of energy (mechanical, electrical, chemical, heat, etc) • An understanding of the maximum efficiency with which we can transform one form of energy into another • Preferred direction by which a system evolves, i.e. will a conversion (reaction) go or not • Understanding of equilibrium • It is not based on the ideas of molecules or atoms. A linkage between these and thermo can be achieved using statistical methods. • It does not tell us about the rate of a process (how fast). Domain of kinetics. 2 CHEM 2880 - Kinetics Surroundings, boundaries, system When considering energy relationships it is important to define your point of reference. 3 CHEM 2880 - Kinetics Types of Systems Open: both mass and Closed: energy can be energy may leave and enter exchanged no matter can enter or leave Isolated: neither mass nor energy can enter or leave. 4 CHEM 2880 - Kinetics Energy Transfer Energy can be transferred between the system and the surroundings as heat (q) or work (w). This leads to a change in the internal energy (E or U) of the system. Heat • the energy transfer that occurs when two bodies at different temperatures come in contact with each other - the hotter body tends to cool while the cooler one warms -

The First Law of Thermodynamics Continued Pre-Reading: §19.5 Where We Are
Lecture 7 The first law of thermodynamics continued Pre-reading: §19.5 Where we are The pressure p, volume V, and temperature T are related by an equation of state. For an ideal gas, pV = nRT = NkT For an ideal gas, the temperature T is is a direct measure of the average kinetic energy of its 3 3 molecules: KE = nRT = NkT tr 2 2 2 3kT 3RT and vrms = (v )av = = r m r M p Where we are We define the internal energy of a system: UKEPE=+∑∑ interaction Random chaotic between atoms motion & molecules For an ideal gas, f UNkT= 2 i.e. the internal energy depends only on its temperature Where we are By considering adding heat to a fixed volume of an ideal gas, we showed f f Q = Nk∆T = nR∆T 2 2 and so, from the definition of heat capacity Q = nC∆T f we have that C = R for any ideal gas. V 2 Change in internal energy: ∆U = nCV ∆T Heat capacity of an ideal gas Now consider adding heat to an ideal gas at constant pressure. By definition, Q = nCp∆T and W = p∆V = nR∆T So from ∆U = Q W − we get nCV ∆T = nCp∆T nR∆T − or Cp = CV + R It takes greater heat input to raise the temperature of a gas a given amount at constant pressure than constant volume YF §19.4 Ratio of heat capacities Look at the ratio of these heat capacities: we have f C = R V 2 and f + 2 C = C + R = R p V 2 so C p γ = > 1 CV 3 For a monatomic gas, CV = R 3 5 2 so Cp = R + R = R 2 2 C 5 R 5 and γ = p = 2 = =1.67 C 3 R 3 YF §19.4 V 2 Problem An ideal gas is enclosed in a cylinder which has a movable piston. -

Adiabatic Bulk Moduli
8.03 at ESG Supplemental Notes Adiabatic Bulk Moduli To find the speed of sound in a gas, or any property of a gas involving elasticity (see the discussion of the Helmholtz oscillator, B&B page 22, or B&B problem 1.6, or French Pages 57-59.), we need the “bulk modulus” of the fluid. This will correspond to the “spring constant” of a spring, and will give the magnitude of the restoring agency (pressure for a gas, force for a spring) in terms of the change in physical dimension (volume for a gas, length for a spring). It turns out to be more useful to use an intensive quantity for the bulk modulus of a gas, so what we want is the change in pressure per fractional change in volume, so the bulk modulus, denoted as κ (the Greek “kappa” which, when written, has a great tendency to look like k, and in fact French uses “K”), is ∆p dp κ = − , or κ = −V . ∆V/V dV The minus sign indicates that for normal fluids (not all are!), a negative change in volume results in an increase in pressure. To find the bulk modulus, we need to know something about the gas and how it behaves. For our purposes, we will need three basic principles (actually 2 1/2) which we get from thermodynamics, or the kinetic theory of gasses. You might well have encountered these in previous classes, such as chemistry. A) The ideal gas law; pV = nRT , with the standard terminology. B) The first law of thermodynamics; dU = dQ − pdV,whereUis internal energy and Q is heat. -

Session 15 Thermodynamics
Session 15 Thermodynamics Cheryl Hurkett Physics Innovations Centre for Excellence in Learning and Teaching CETL (Leicester) Department of Physics and Astronomy University of Leicester 1 Contents Welcome .................................................................................................................................. 4 Session Authors .................................................................................................................. 4 Learning Objectives .............................................................................................................. 5 The Problem ........................................................................................................................... 6 Issues .................................................................................................................................... 6 Energy, Heat and Work ........................................................................................................ 7 The First Law of thermodynamics................................................................................... 8 Work..................................................................................................................................... 9 Heat .................................................................................................................................... 11 Principal Specific heats of a gas ..................................................................................... 12 Summary .......................................................................................................................... -

Thermodynamic Potentials
THERMODYNAMIC POTENTIALS, PHASE TRANSITION AND LOW TEMPERATURE PHYSICS Syllabus: Unit II - Thermodynamic potentials : Internal Energy; Enthalpy; Helmholtz free energy; Gibbs free energy and their significance; Maxwell's thermodynamic relations (using thermodynamic potentials) and their significance; TdS relations; Energy equations and Heat Capacity equations; Third law of thermodynamics (Nernst Heat theorem) Thermodynamics deals with the conversion of heat energy to other forms of energy or vice versa in general. A thermodynamic system is the quantity of matter under study which is in general macroscopic in nature. Examples: Gas, vapour, vapour in contact with liquid etc.. Thermodynamic stateor condition of a system is one which is described by the macroscopic physical quantities like pressure (P), volume (V), temperature (T) and entropy (S). The physical quantities like P, V, T and S are called thermodynamic variables. Any two of these variables are independent variables and the rest are dependent variables. A general relation between the thermodynamic variables is called equation of state. The relation between the variables that describe a thermodynamic system are given by first and second law of thermodynamics. According to first law of thermodynamics, when a substance absorbs an amount of heat dQ at constant pressure P, its internal energy increases by dU and the substance does work dW by increase in its volume by dV. Mathematically it is represented by 풅푸 = 풅푼 + 풅푾 = 풅푼 + 푷 풅푽….(1) If a substance absorbs an amount of heat dQ at a temperature T and if all changes that take place are perfectly reversible, then the change in entropy from the second 풅푸 law of thermodynamics is 풅푺 = or 풅푸 = 푻 풅푺….(2) 푻 From equations (1) and (2), 푻 풅푺 = 풅푼 + 푷 풅푽 This is the basic equation that connects the first and second laws of thermodynamics. -

1 Microphysics
ASTR 501 Stellar Physics 1 1 Microphysics • equation of state, density: ρ(P, T, X) • radiative absorption coefficient, opacity: κ(P, T, X) • rate of energy production: (P, T, X) (nuclear physics) Nuclear physics also responsible for composition changes dXj = Fˆj · Xj (1) dt burn while the total composition change also includes a mixing term: dX ∂ dX i = 4πr2ρ D i (2) dt mix ∂m dm 1.1 Equation of state For the fluid to have an EOS it must be collisional: lregion λ (3) where λ is the mean free path. P from equation of state, consider three cases: ASTR 501 Stellar Physics 2 (a) ideal gas equation of state: p = R ρT (b) barotropic equation of state: pressure p depends only on ρ, as for example • isothermal: p ∼ ρ • adiabatic: p = Kργ Plus non-ideal effects, e.g. electron degeneracy. 1.2 Ideal gas equation of state Assumption: internal energy entirely in kinetic energy according to kinetic theory: = CVT , where CV is the specific heat at constant volume. This can be seen from the first law of thermodynamics dQ = d + pdV , where V is the specific volume, d if we express d = dT dT . d Similarly, the specific heat at constant pressure Cp = dT + R where R is the gas constant. This follows from the ideal gas equation of state. [†13] It also follows that Cp − CV = R [†14], and the ratio of specific heats is defined as C γ = p . (4) CV ASTR 501 Stellar Physics 3 α CV can be related to R as CV = 2 R , where α is the number of degrees of freedom 1 per particle, since the energy per particle per degree of freedom is 2kT where k is 2+α the Boltzmann constant, and R = nk. -

THERMODYNAMICS Entropy: Entropy Is Defined As a Quantitative Measure of Disorder Or Randomness in a System. the Heat Change, Dq
Produced with a Trial Version of PDF Annotator - www.PDFAnnotator.com THERMODYNAMICS Entropy: Entropy is defined as a quantitative measure of disorder or randomness in a system. The heat change, dq and the temperature T are thermodynamic quantities. A thermodynamic function whose change (dq/T) is independent of the path of the system is called entropy 푑푆 = 푑푞 푇 1. Entropy is a state function whose magnitude depends only on the parameters of the system and can be expressed in terms of (P,V,T) 2. dS is a perfect differential. Its value depends only on the initial and final states of the system 3. Absorption of heat increases entropy of the system. In a reversible adiabatic change dq=0, the entropy change is zero 4. For carnot cycle ∮ 푑푆 = 0 5. The net entropy change in a reversible process is zero, ∆푆푢푛푖푣푒푟푠푒 = ∆푆푠푦푠푡푒푚 + ∆푆푠푢푟푟표푢푛푑푖푛푔 = 0 In irreversible expansion ∆푆푢푛푖푣 = +푣푒 cyclic processs, ∆푆푢푛푖푣 > 0 All natural process will take place in a direction in which the entropy would increase. A thermodynamically irreversible process is always accompanied by an increase in the entropy of the system and its surroundings taken together while in a thermodynamically reversible process, the entropy of the system and its surroundings taken together remains constant. Entropy change in isothermal and reversible expansion of ideal gas: In isothermal expansion of an ideal gas carried out reversibly, there will be no change in internal energy, ∆U=0, hence from the first law q = -W In such case, the work done in the expansion of n moles of a gas from volume V1 to V2 at constant temperature T is given by – 푊 = 푛푅푇푙푛(푉2) 푉1 푉2 푞푟푒푣 = −푊 = 푛푅푇푙푛( ) 푉1 Hence 푑푆 = 푑푞 = 1 푛푅푇푙푛 (푉2) = 푛푅푙푛 (푉2) 푇 푇 푉1 푉1 (Problem: 5 moles of ideal gas expand reversibly from volume of 8 dm3 to 80 dm3 at a temperature of 27oC. -

12/8 and 12/10/2010
PY105 C1 1. Help for Final exam has been posted on WebAssign. 2. The Final exam will be on Wednesday December 15th from 6-8 pm. First Law of Thermodynamics 3. You will take the exam in multiple rooms, divided as follows: SCI 107: Abbasi to Fasullo, as well as Khajah PHO 203: Flynn to Okuda, except for Khajah SCI B58: Ordonez to Zhang 1 2 Heat and Work done by a Gas Thermodynamics Initial: Consider a cylinder of ideal Thermodynamics is the study of systems involving gas at room temperature. Suppose the piston on top of energy in the form of heat and work. the cylinder is free to move vertically without friction. When the cylinder is placed in a container of hot water, heat Equilibrium: is transferred into the cylinder. Where does the heat energy go? Why does the volume increase? 3 4 The First Law of Thermodynamics The First Law of Thermodynamics The First Law is often written as: Some of the heat energy goes into raising the temperature of the gas (which is equivalent to raising the internal energy of the gas). The rest of it does work by raising the piston. ΔEQWint =− Conservation of energy leads to: QEW=Δ + int (the first law of thermodynamics) This form of the First Law says that the change in internal energy of a system is Q is the heat added to a system (or removed if it is negative) equal to the heat supplied to the system Eint is the internal energy of the system (the energy minus the work done by the system (usually associated with the motion of the atoms and/or molecules).