Assessing Landscape Effects on Genetics and Dispersal of the Rocky Mountain Apollo Butterfly Arnassiusp Smintheus Using a Resistance Mapping Approach
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Large-Scale Movement Behavior in a Reintroduced Predator Population
doi: 10.1111/ecog.03030 41 126–139 ECOGRAPHY Research Large-scale movement behavior in a reintroduced predator population Frances E. Buderman, Mevin B. Hooten, Jacob S. Ivan and Tanya M. Shenk F. E. Buderman (http://orcid.org/0000-0001-9778-9906) ([email protected]) Dept of Fish, Wildlife, and Conservation Biology, Colorado State Univ., Fort Collins, CO, USA. – M. B. Hooten, U.S. Geological Survey, Colorado Cooperative Fish and Wildlife Research Unit, Dept of Fish, Wildlife, and Conservation Biology and Statistics, Colorado State Univ., Fort Collins, CO, USA, and Graduate Degree Program in Ecology, Colorado State Univ., Fort Collins, CO, USA. – J. S. Ivan, Colorado Parks and Wildlife, Fort Collins, CO, USA. – T. M. Shenk, National Park Service, Great Plains Cooperative Ecosystem Studies Unit, Univ. of Nebraska, Lincoln, NE, USA. Ecography Understanding movement behavior and identifying areas of landscape connectivity is 41: 126–139, 2018 critical for the conservation of many species. However, collecting fine-scale movement doi: 10.1111/ecog.03030 data can be prohibitively time consuming and costly, especially for rare or endangered species, whereas existing data sets may provide the best available information on animal Subject Editor: Timothy Keitt. movement. Contemporary movement models may not be an option for modeling Editor-in-Chief: Miguel Araújo. existing data due to low temporal resolution and large or unusual error structures, but Accepted 17 July 2017 inference can still be obtained using a functional movement modeling approach. We use a functional movement model to perform a population-level analysis of telemetry data collected during the reintroduction of Canada lynx to Colorado. -

Ours to Save: the Distribution, Status & Conservation Needs of Canada's Endemic Species
Ours to Save The distribution, status & conservation needs of Canada’s endemic species June 4, 2020 Version 1.0 Ours to Save: The distribution, status & conservation needs of Canada’s endemic species Additional information and updates to the report can be found at the project website: natureconservancy.ca/ourstosave Suggested citation: Enns, Amie, Dan Kraus and Andrea Hebb. 2020. Ours to save: the distribution, status and conservation needs of Canada’s endemic species. NatureServe Canada and Nature Conservancy of Canada. Report prepared by Amie Enns (NatureServe Canada) and Dan Kraus (Nature Conservancy of Canada). Mapping and analysis by Andrea Hebb (Nature Conservancy of Canada). Cover photo credits (l-r): Wood Bison, canadianosprey, iNaturalist; Yukon Draba, Sean Blaney, iNaturalist; Salt Marsh Copper, Colin Jones, iNaturalist About NatureServe Canada A registered Canadian charity, NatureServe Canada and its network of Canadian Conservation Data Centres (CDCs) work together and with other government and non-government organizations to develop, manage, and distribute authoritative knowledge regarding Canada’s plants, animals, and ecosystems. NatureServe Canada and the Canadian CDCs are members of the international NatureServe Network, spanning over 80 CDCs in the Americas. NatureServe Canada is the Canadian affiliate of NatureServe, based in Arlington, Virginia, which provides scientific and technical support to the international network. About the Nature Conservancy of Canada The Nature Conservancy of Canada (NCC) works to protect our country’s most precious natural places. Proudly Canadian, we empower people to safeguard the lands and waters that sustain life. Since 1962, NCC and its partners have helped to protect 14 million hectares (35 million acres), coast to coast to coast. -

Anthropogenic Threats to High-Altitude Parnassian Diversity Fabien Condamine, Felix Sperling
Anthropogenic threats to high-altitude parnassian diversity Fabien Condamine, Felix Sperling To cite this version: Fabien Condamine, Felix Sperling. Anthropogenic threats to high-altitude parnassian diversity. News of The Lepidopterists’ Society, 2018, 60, pp.94-99. hal-02323624 HAL Id: hal-02323624 https://hal.archives-ouvertes.fr/hal-02323624 Submitted on 23 Oct 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. _______________________________________________________________________________________News of The Lepidopterists’ Society Volume 60, Number 2 Conservation Matters: Contributions from the Conservation Committee Anthropogenic threats to high-altitude parnassian diversity Fabien L. Condamine1 and Felix A.H. Sperling2 1CNRS, UMR 5554 Institut des Sciences de l’Evolution (Université de Montpellier), Place Eugène Bataillon, 34095 Montpellier, France [email protected], corresponding author 2Department of Biological Sciences, University of Alberta, Edmonton T6G 2E9, Alberta, Canada Introduction extents (Chen et al. 2011). However, this did not result in increases in range area because the area of land available Global mean annual temperatures increased by ~0.85° declines with increasing elevation. Accordingly, extinc- C between 1880 and 2012 and are likely to rise by an tion risk may increase long before species reach a summit, additional 1° C to 4° C by 2100 (Stocker et al. -

Yukon Butterflies a Guide to Yukon Butterflies
Wildlife Viewing Yukon butterflies A guide to Yukon butterflies Where to find them Currently, about 91 species of butterflies, representing five families, are known from Yukon, but scientists expect to discover more. Finding butterflies in Yukon is easy. Just look in any natural, open area on a warm, sunny day. Two excellent butterfly viewing spots are Keno Hill and the Blackstone Uplands. Pick up Yukon’s Wildlife Viewing Guide to find these and other wildlife viewing hotspots. Visitors follow an old mining road Viewing tips to explore the alpine on top of Keno Hill. This booklet will help you view and identify some of the more common butterflies, and a few distinctive but less common species. Additional species are mentioned but not illustrated. In some cases, © Government of Yukon 2019 you will need a detailed book, such as , ISBN 978-1-55362-862-2 The Butterflies of Canada to identify the exact species that you have seen. All photos by Crispin Guppy except as follows: In the Alpine (p.ii) Some Yukon butterflies, by Ryan Agar; Cerisy’s Sphynx moth (p.2) by Sara Nielsen; Anicia such as the large swallowtails, Checkerspot (p.2) by Bruce Bennett; swallowtails (p.3) by Bruce are bright to advertise their Bennett; Freija Fritillary (p.12) by Sonja Stange; Gallium Sphinx presence to mates. Others are caterpillar (p.19) by William Kleeden (www.yukonexplorer.com); coloured in dull earth tones Butterfly hike at Keno (p.21) by Peter Long; Alpine Interpretive that allow them to hide from bird Centre (p.22) by Bruce Bennett. -
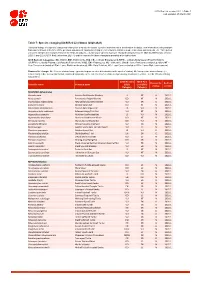
Table 7: Species Changing IUCN Red List Status (2020-2021)
IUCN Red List version 2021-1: Table 7 Last Updated: 25 March 2021 Table 7: Species changing IUCN Red List Status (2020-2021) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2020 (IUCN Red List version 2020-3) and 2021 (IUCN Red List version 2021-1) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2020) List (2021) change version Category -

Rationales for Animal Species Considered for Designation As Species of Conservation Concern Inyo National Forest
Rationales for Animal Species Considered for Designation as Species of Conservation Concern Inyo National Forest Prepared by: Wildlife Biologists and Natural Resources Specialist Regional Office, Inyo National Forest, and Washington Office Enterprise Program for: Inyo National Forest August 2018 1 In accordance with Federal civil rights law and U.S. Department of Agriculture (USDA) civil rights regulations and policies, the USDA, its Agencies, offices, and employees, and institutions participating in or administering USDA programs are prohibited from discriminating based on race, color, national origin, religion, sex, gender identity (including gender expression), sexual orientation, disability, age, marital status, family/parental status, income derived from a public assistance program, political beliefs, or reprisal or retaliation for prior civil rights activity, in any program or activity conducted or funded by USDA (not all bases apply to all programs). Remedies and complaint filing deadlines vary by program or incident. Persons with disabilities who require alternative means of communication for program information (e.g., Braille, large print, audiotape, American Sign Language, etc.) should contact the responsible Agency or USDA’s TARGET Center at (202) 720-2600 (voice and TTY) or contact USDA through the Federal Relay Service at (800) 877-8339. Additionally, program information may be made available in languages other than English. To file a program discrimination complaint, complete the USDA Program Discrimination Complaint Form, AD-3027, found online at http://www.ascr.usda.gov/complaint_filing_cust.html and at any USDA office or write a letter addressed to USDA and provide in the letter all of the information requested in the form. To request a copy of the complaint form, call (866) 632-9992. -
![Effects of Canopy Coverage on the Immature Stages of the Clouded Apollo Butterfly [Parnassius Mnemosyne (L.)] with Observations on Larval Behaviour](https://docslib.b-cdn.net/cover/7545/effects-of-canopy-coverage-on-the-immature-stages-of-the-clouded-apollo-butterfly-parnassius-mnemosyne-l-with-observations-on-larval-behaviour-2307545.webp)
Effects of Canopy Coverage on the Immature Stages of the Clouded Apollo Butterfly [Parnassius Mnemosyne (L.)] with Observations on Larval Behaviour
© Entomologica Fennica. 17 June 2005 Effects of canopy coverage on the immature stages of the Clouded Apollo butterfly [Parnassius mnemosyne (L.)] with observations on larval behaviour Panu Välimäki & Juhani Itämies Välimäki, P. & Itämies, J. 2005: Effects of canopy coverage on the immature stages of the Clouded Apollo butterfly [Parnassius mnemosyne (L.)] with obser- vations on larval behaviour. — Entomol. Fennica 16: 117–123. The immature stages of the Clouded Apollo (Parnassius mnemosyne)areas- sumed to be thermophilic due to the possible time limitations and variable weather conditions during their development. Thus, the degree of canopy cover- age may affect habitat use by the species. We explored the spatial distribution of larvae and the development time of pupae under variable canopy coverage condi- tions. Larvae were most abundant in the areas exposed to direct sunlight, al- though the last instar larvae are mobile. Larvae also basked under litter between their foraging periods, probably to enhance digestion and the food intake rate. Moreover, pupal development was retarded by increasing canopy coverage. Pro- longed pupal development and larval avoidance of Corydalis growths under tree canopies indicate that the species suffers from overgrowing and consequently in- creasing canopy coverage. P. Välimäki & J. Itämies, University of Oulu, Department of Zoology, P. O. Box 3000, FI-90014 University of Oulu, Finland; E-mail: [email protected], [email protected] Received 28 May 2003, accepted 13 February 2004 1. Introduction nature conservation act in 1976 [Maa- ja metsä- talousministeriö (1976), see also Mikkola & The Clouded Apollo butterfly [Parnassius Häkkinen (1977)]. However, effective conserva- mnemosyne (L.)] is considered a threatened spe- tion is not possible if the biology of the species is cies in northern and central Europe (Heath 1981, not adequately known. -

A SKELETON CHECKLIST of the BUTTERFLIES of the UNITED STATES and CANADA Preparatory to Publication of the Catalogue Jonathan P
A SKELETON CHECKLIST OF THE BUTTERFLIES OF THE UNITED STATES AND CANADA Preparatory to publication of the Catalogue © Jonathan P. Pelham August 2006 Superfamily HESPERIOIDEA Latreille, 1809 Family Hesperiidae Latreille, 1809 Subfamily Eudaminae Mabille, 1877 PHOCIDES Hübner, [1819] = Erycides Hübner, [1819] = Dysenius Scudder, 1872 *1. Phocides pigmalion (Cramer, 1779) = tenuistriga Mabille & Boullet, 1912 a. Phocides pigmalion okeechobee (Worthington, 1881) 2. Phocides belus (Godman and Salvin, 1890) *3. Phocides polybius (Fabricius, 1793) =‡palemon (Cramer, 1777) Homonym = cruentus Hübner, [1819] = palaemonides Röber, 1925 = ab. ‡"gunderi" R. C. Williams & Bell, 1931 a. Phocides polybius lilea (Reakirt, [1867]) = albicilla (Herrich-Schäffer, 1869) = socius (Butler & Druce, 1872) =‡cruentus (Scudder, 1872) Homonym = sanguinea (Scudder, 1872) = imbreus (Plötz, 1879) = spurius (Mabille, 1880) = decolor (Mabille, 1880) = albiciliata Röber, 1925 PROTEIDES Hübner, [1819] = Dicranaspis Mabille, [1879] 4. Proteides mercurius (Fabricius, 1787) a. Proteides mercurius mercurius (Fabricius, 1787) =‡idas (Cramer, 1779) Homonym b. Proteides mercurius sanantonio (Lucas, 1857) EPARGYREUS Hübner, [1819] = Eridamus Burmeister, 1875 5. Epargyreus zestos (Geyer, 1832) a. Epargyreus zestos zestos (Geyer, 1832) = oberon (Worthington, 1881) = arsaces Mabille, 1903 6. Epargyreus clarus (Cramer, 1775) a. Epargyreus clarus clarus (Cramer, 1775) =‡tityrus (Fabricius, 1775) Homonym = argentosus Hayward, 1933 = argenteola (Matsumura, 1940) = ab. ‡"obliteratus" -

Pleistocene Evolutionary History of the Clouded Apollo (Parnassius
Molecular Ecology (2008) 17, 4248–4262 doi: 10.1111/j.1365-294X.2008.03901.x PleistoceneBlackwell Publishing Ltd evolutionary history of the Clouded Apollo (Parnassius mnemosyne): genetic signatures of climate cycles and a ‘time-dependent’ mitochondrial substitution rate P. GRATTON,* M. K. KONOPI5SKI† and V. SBORDONI* *Department of Biology, University of Tor Vergata, Via Cracovia 1, 00133 Roma, Italy, †Institute of Nature Conservation PAS, al. Mickiewicza 33, 31–120 Kraków, Poland Abstract Genetic data are currently providing a large amount of new information on past distribution of species and are contributing to a new vision of Pleistocene ice ages. Nonetheless, an increasing number of studies on the ‘time dependency’ of mutation rates suggest that date assessments for evolutionary events of the Pleistocene might be overestimated. We analysed mitochondrial (mt) DNA (COI) sequence variation in 225 Parnassius mnemosyne individuals sampled across central and eastern Europe in order to assess (i) the existence of genetic signatures of Pleistocene climate shifts; and (ii) the timescale of demographic and evolutionary events. Our analyses reveal a phylogeographical pattern markedly influenced by the Pleis- tocene/Holocene climate shifts. Eastern Alpine and Balkan populations display compara- tively high mtDNA diversity, suggesting multiple glacial refugia. On the other hand, three widely distributed and spatially segregated lineages occupy most of northern and eastern Europe, indicating postglacial recolonization from different refugial areas. We show that a conventional ‘phylogenetic’ substitution rate cannot account for the present distribution of genetic variation in this species, and we combine phylogeographical pattern and palaeoeco- logical information in order to determine a suitable intraspecific rate through a Bayesian coalescent approach. -

Effects of Experimental Population Extinction for the Spatial Population Dynamics of the Butterfl Y Parnassius Smintheus
Oikos 119: 1961–1969, 2010 doi: 10.1111/j.1600-0706.2010.18666.x © 2010 e Authors. Oikos © 2010 Nordic Society Oikos Subject Editor: Daniel Gruner. Accepted 26 April 2010 Effects of experimental population extinction for the spatial population dynamics of the butterfl y Parnassius smintheus Stephen F. Matter and Jens Roland S. F. Matter ([email protected]), Dept of Biological Sciences, Univ. of Cincinnati, Cincinnati, OH 45220, USA. – J. Roland, Dept of Biological Sciences, Univ. of Alberta, Edmonton, AB T5K 1X4, Canada. While many studies have examined factors potentially impacting the rate of local population extinction, few experimental studies have examined the consequences of extinction for spatial population dynamics. Here we report results from a large- scale, long-term experiment examining the e ects of local population extinction for the dynamics of surrounding popula- tions. From 2001 – 2008 we removed all adult butterfl ies from two large, neighboring populations within a system of 17 subpopulations of the Rocky Mountain Apollo butterfl y, Parnassius smintheus . Surrounding populations were monitored using individual, mark–recapture methods. We found that population removal decreased immigration to surrounding populations in proportion to their connectivity to the removed populations. Correspondingly, within-generation popula- tion abundance declined. Despite these e ects, we saw little consistent impact between generations. e extinction rates of surrounding populations were una ected and local population growth was not consistently reduced by the lack of immigra- tion. e broader results show that immigration a ects local abundance within generations, but dynamics are mediated by density-dependence within populations and by broader density-independent factors acting between generations. -

The Coleopteran Fauna of Sultan Creek-Molas Lake Area with Special Emphasis on Carabidae and How the Geological Bedrock Influenc
THE COLEOPTERAN FAUNA OF SULTAN CREEK-MOLAS LAKE AREA WITH SPECIAL EMPHASIS ON CARABIDAE AND HOW THE GEOLOGICAL BEDROCK INFLUENCES BIODIVERSITY AND COMMUNITY STRUCTURE IN THE SAN JUAN MOUNTAINS, SAN JUAN COUNTY, COLORADO Melanie L. Bergolc A Dissertation Submitted to the Graduate College of Bowling Green State University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY August 2009 Committee: Daniel Pavuk, Advisor Kurt Panter Graduate Faculty Representative Jeff Holland Rex Lowe Moira van Staaden © 2009 Melanie L. Bergolc All Rights Reserved iii ABSTRACT Daniel Pavuk, Advisor Few studies have been performed on coleopteran (beetle) biodiversity in mountain ecosystems and relating them to multiple environmental factors. None of the studies have examined geologic influences on beetle communities. Little coleopteran research has been performed in the Colorado Rocky Mountains. The main objectives of this study were to catalog the coleopteran fauna of a subalpine meadow in the San Juan Mountains of Colorado and investigate the role geology had in the community structure of the Carabidae (ground beetles). The study site, a 160,000 m2 plot, was located near Sultan Creek and Molas Lake in San Juan County, Colorado. Five sites were in each bedrock formation: Molas, Elbert, and Ouray-Leadville. Insects were collected via pitfall trapping in 2006 and 2007, and identified by comparison with museum specimens, museum and insect identification websites, and by taxonomic experts. Biological and physical factors were recorded for each site: detritus cover and weight, plant cover and height, plant species richness, aspect, elevation, slope, soil temperature, pH, moisture, and compressive strength, and sediment size distribution. -

AC18 Doc. 8.1
AC18 Doc. 8.1 CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ___________________ Eighteenth meeting of the Animals Committee San José (Costa Rica), 8-12 April 2002 Periodic review of animal taxa in the Appendices (Resolution Conf. 11.1) REPORT OF THE WORKING GROUP This document has been prepared by the Chairman of the working group on review of animal taxa in the Appendices. Introduction 1. The North American Regional Representative has been coordinating an intersessional working group on review of animal taxa in the Appendices, with the goal of making significant progress on tasks resulting from deliberations at AC 17. Tasks identified at AC 17 included: (a) the completion of various taxon reviews identified at AC 15 and AC 16; (b) development of guidelines for conducting reviews of animal taxa in the Appendices, (c) development of a rapid assessment technique for screening multiple taxa (or higher-order taxa) at one time; and (d) evaluation of crocodile ranching operations in the framework of the Review of the Appendices. Remaining Species from AC 15 through AC 16 2. At the conclusion of AC17, 11 species reviews remained to be completed, and one preliminary draft review (Cnemidophorus hyperythrus) was identified for revision (Table 1). In the period since AC17, the review of Cnemidophorus hyperythrus has been completed, and two of the other species reviews have been completed (Anas aucklandica, Parnassius apollo). This low completion percentage is one drawback to the voluntary nature of the review process, and lends support to the need to look at alternative approaches for getting reviews done.