Translocation of the Endangered Apollo Butterfly Parnassius Apollo in Southern Finland
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Assessing Landscape Effects on Genetics and Dispersal of the Rocky Mountain Apollo Butterfly Arnassiusp Smintheus Using a Resistance Mapping Approach
Western University Scholarship@Western Electronic Thesis and Dissertation Repository 10-27-2017 10:30 AM Assessing Landscape Effects on Genetics and Dispersal of the Rocky Mountain Apollo Butterfly arnassiusP smintheus using a Resistance Mapping Approach Ning Chen The University of Western Ontario Supervisor Dr. Nusha Keyghobadi The University of Western Ontario Graduate Program in Biology A thesis submitted in partial fulfillment of the equirr ements for the degree in Master of Science © Ning Chen 2017 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Biology Commons Recommended Citation Chen, Ning, "Assessing Landscape Effects on Genetics and Dispersal of the Rocky Mountain Apollo Butterfly Parnassius smintheus using a Resistance Mapping Approach" (2017). Electronic Thesis and Dissertation Repository. 5058. https://ir.lib.uwo.ca/etd/5058 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. Abstract Landscape variables that best explain genetic differentiation may not also best explain dispersal patterns, but many studies use genetic differentiation as a proxy for dispersal. I tested the effects of landscape on both genetic differentiation and dispersal in parallel, to explore whether landscape effects on genetic differentiation between populations and landscape effects on dispersal would be comparable in such contexts. I used circuit theory (Circuitscape) and least cost transect analysis to evaluate the effects of landscape on both movement and genetic differentiation of the butterfly, Parnassius smintheus, in the Jumpingpound Ridge study system. -

Spotting Butterflies Says Ulsh
New & Features “A butterfly’s lifespan generally cor- responds with the size of the butterfly,” Spotting Butterflies says Ulsh. The tiny blues often seen in the mountains generally only live about How, when and where to find Lepidoptera in 10 days. Some species, however, will the Cascades and Olympics overwinter in the egg, pupa or chrysalid form (in the cocoon prior to becoming winged adults). A few Northwest spe- cies overwinter as adults, and one—the mourning cloak—lives for ten months, and is the longest lived butterfly in North America. The first thing that butterflies do upon emerging from the chrysalis and unfold- ing their wings is to breed. In their search for mates, some butterflies “hilltop,” or stake out spots on high trees or ridgelines to make themselves more prominent. A butterfly’s wing colors serve two distinct purposes. The dorsal, or upperside of the wings, are colorful, and serve to attract mates. The ventral, or underside of the wings generally serves to camouflage the insects. So a butterfly such as the satyr comma has brilliant orange and yellow Western tiger and pale tiger swallowtail butterflies “puddling.” When looking for for spots when seen with wings open, and butterflies along the trail keep an eye on moist areas or meadows with flowers. a bark-like texture to confuse predators when its wings are closed. By Andrew Engelson Butterfly Association, about where, how As adults, butterflies also seek out Photos by Idie Ulsh and when to look for butterflies in our nectar and water. Butterflies generally mountains. Ulsh is an accomplished Butterflies are the teasers of wildlife. -

Anthropogenic Threats to High-Altitude Parnassian Diversity Fabien Condamine, Felix Sperling
Anthropogenic threats to high-altitude parnassian diversity Fabien Condamine, Felix Sperling To cite this version: Fabien Condamine, Felix Sperling. Anthropogenic threats to high-altitude parnassian diversity. News of The Lepidopterists’ Society, 2018, 60, pp.94-99. hal-02323624 HAL Id: hal-02323624 https://hal.archives-ouvertes.fr/hal-02323624 Submitted on 23 Oct 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. _______________________________________________________________________________________News of The Lepidopterists’ Society Volume 60, Number 2 Conservation Matters: Contributions from the Conservation Committee Anthropogenic threats to high-altitude parnassian diversity Fabien L. Condamine1 and Felix A.H. Sperling2 1CNRS, UMR 5554 Institut des Sciences de l’Evolution (Université de Montpellier), Place Eugène Bataillon, 34095 Montpellier, France [email protected], corresponding author 2Department of Biological Sciences, University of Alberta, Edmonton T6G 2E9, Alberta, Canada Introduction extents (Chen et al. 2011). However, this did not result in increases in range area because the area of land available Global mean annual temperatures increased by ~0.85° declines with increasing elevation. Accordingly, extinc- C between 1880 and 2012 and are likely to rise by an tion risk may increase long before species reach a summit, additional 1° C to 4° C by 2100 (Stocker et al. -

Phylogeography of Koramius Charltonius (Gray, 1853) (Lepidoptera: Papilionidae): a Case of Too Many Poorly Circumscribed Subspecies
©Societas Europaea Lepidopterologica; download unter http://www.soceurlep.eu/ und www.zobodat.at Nota Lepi. 39(2) 2016: 169–191 | DOI 10.3897/nl.39.7682 Phylogeography of Koramius charltonius (Gray, 1853) (Lepidoptera: Papilionidae): a case of too many poorly circumscribed subspecies Stanislav K. Korb1, Zdenek F. Fric2, Alena Bartonova2 1 Institute of Biology, Komi Science Center, Ural Division of the Russian Academy of Sciences, Kommunisticheskaya 28, 167982 Syktyvkar, Russia; [email protected] 2 Institute of Entomology, Biology Centre, Academy of Sciences of the Czech Republic, Branisovska 31, 370 05 Ceske Budejovice, Czech Republic; [email protected] http://zoobank.org/670C4AB9-A810-4F7E-A3FC-B433940CFD67 Received 2 January 2016; accepted 3 October 2016; published: 27 October 2016 Subject Editor: Thomas Schmitt. Abstract. Koramius charltonius (Gray, 1853) (Lepidoptera: Papilionidae) is distributed in the mountains of Central Asia. We analysed genetic and phylogeographic patterns throughout the western part of its range using a mitochondrial marker (COI). We also analysed the wing pattern using multivariate statistics. We found that the species contains several unique haplotypes in the west and shared haplotypes in the east. The haplotype groups do not correspond to the wing pattern and also the described subspecies do not correspond to either the haplotypes or the groups circumscribed by the wing pattern. Currently, there are more than ten subspecies of K. charltonius in Central Asia; based on our analyses we suggest a reduction to only five of them. The fol- lowing nomenclatural changes are applied: (1) K. charltonius aenigma Dubatolov & Milko, 2003, syn. n., K. charltonius sochivkoi Churkin, 2009, syn.n., and K. -
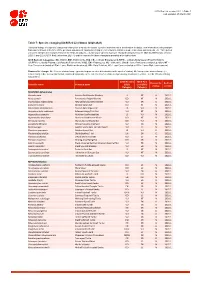
Table 7: Species Changing IUCN Red List Status (2020-2021)
IUCN Red List version 2021-1: Table 7 Last Updated: 25 March 2021 Table 7: Species changing IUCN Red List Status (2020-2021) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2020 (IUCN Red List version 2020-3) and 2021 (IUCN Red List version 2021-1) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2020) List (2021) change version Category -

Ts Denver Museum of Nature & Science Reports
DENVER MUSEUM OF NATURE & SCIENCE REPORTS DENVER MUSEUM OF NATURE & SCIENCE REPORTS DENVER MUSEUM OF NATURE & SCIENCE & SCIENCE OF NATURE DENVER MUSEUM NUMBER 16, OCTOBER 11, 2019 SCIENCE.DMNS.ORG/MUSEUM-PUBLICATIONS Denver Museum of Nature & Science Reports 2001 Colorado Boulevard (Print) ISSN 2374-7730 Denver, CO 80205, U.S.A. Denver Museum of Nature & Science Reports (Online) ISSN 2374-7749 REPORTS • NUMBER 16 • OCTOBER 11, 2019 • NUMBER 16 OCTOBER Cover photo: Oreas Anglewing (Polygonia oreas nigrozephyrus Scott, 1984), Gregory Canyon, Boulder County, Colorado, USA, 2 October 1973, leg. Michael G. Pogue. Photo: Bob Livingston. The Denver Museum of Nature & Science Reports (ISSN Frank Krell, PhD, Editor and Production 2374-7730 [print], ISSN 2374-7749 [online]) is an open- access, non peer-reviewed scientifi c journal publishing papers about DMNS research, collections, or other Program and Abstracts Museum related topics, generally authored or co-authored 30th Annual Meeting by Museum staff or associates. Peer review will only be arranged on request of the authors. of the High Country Lepidopterists October 11–12, 2019 The journal is available online at science.dmns.org/ Museum-Publications free of charge. Paper copies Denver Museum of Nature & Science are available for purchase from our print-on-demand publisher Lulu (www.lulu.com). DMNS owns the copyright of the works published in the Reports, which are Frank-Thorsten Krell (Ed.) published under the Creative Commons Attribution Non- Commercial license. For commercial use of published -

Lepidoptera) (Zweiter Teil) 1)
ΡARNASSIANA NOVA XLIX DIE ARTEN UND UNTERARTEN DER PARNASSIIDAE (LEPIDOPTERA) (ZWEITER TEIL) 1) von CURT EISNER Rijksmuseum van Natuurlijke Historie, Leiden Mit zwei Tafeln Parnassius honrathi honrathi Staudinger Zugänge: SultanHazreth Geb. 1 ♂ 1 ♀; Sarafschan 3 ♂; SultanHazreth Geb. 2 ♂ 1 ♀; Sarafschan 1 ♂ 1 ♀; Kargaisk, KandykTau 1 ♂, f. costalis nigroocellata n.c. 1 ♂; Samarkand 1 ♂ 2 ♀; Barschepky 1 ♂; Sultan HazrethGeb., f. nigroocellata n.c. 1 ♂; Samarkand 1 ♂; Karategin 1 ♂; Pamir(?) 1 ♂; SultanHazreth Geb. 1 ♀. Sie bestätigen die Merkmale dieser markanten Unterart. Parnassius honrathi ernesti Bryk Zugänge: WestPamir 1 ♂ 1 ♀; Karategin 2 ♂; WestPamir 1 ♂; Tur kestan (?) 1 ♀; Garm 1 ♂; WestPamir 1 ♂ 1 ♀; Garm, f. nigroocellata n.c. + inpicta n.c. 1 ♂, 2 ♀; Pamir(?) 1 ♂ 1 ♀; Garm 1 ♂. Bezüglich der vagen Fundortangaben und der damit zusammenhängenden Schwierigkeit der Einordnung verweise ich auf das darüber in 1950 (Parn. Nov. xxix: 144) Ausgeführte. Allgemein lässt sich aus dem mir vorliegenden Material von honrathi Staudinger sagen, dass seine Vertreter im Westen des Fluggebietes am dunkelsten und kräftigsten gezeichnet auftreten und nach Osten zu einen helleren Habitus zeigen. Parnassius honrathi alburnus Stichel Zugänge: Chorog 3 ♂ 2 ♀, ex c. Sheljuzhko; Pamir(?) 1 ♂ 1 ♀; Chorog 1 ♂ 1 ♀, ex c. Sheljuzhko; Chorog 7 ♂ 3 ♀, f. posteriorsubmarginalis extenta (= Submarginale wurzelwärts in ein bis zu den Costalflecken und 1) Zu Erster Teil (Zool. Verh., 135) : Aus der Arbeit von P. R. Ackery "A guide to the genera and species of Parnassiinae (Lepidoptera: Papilionidae)" (1975, Bull. Brit. Mus. (Nat. Hist.), Entom., 31(4)) habe ich entnommen, dass im ersten Teil dieser Arbeit (Parn. Nov. xlix) zwei der dort angeführten Namen Synonyma sind: Parnassius clarius Eversmann (p. -

Papilio (New Series) #12, Some O
(NEW Dec. 3, PAPILIO SERIES) 2008 GEOGRAPHIC VARIATION AND NEW TAXA OF WESTERN NORTH AMERICAN BUTTERFLIES, ESPECIALLY FROM COLORADO By James A. Scott & Michaels. Fisher, with some parts by David M. Wright, Stephen M. Spomer, Norbert G. Kondla, Todd Stout, Matthew C. Garhart, & Gary M. Marrone Introduction and Abstract Michael Fisher is currently updating the 1957 book Colorado Butterflies, by F. Martin Brown, J. Donald Eff, and Bernard Rotger (Fisher 2005a, 2005b, 2006). This project has emphasized the necessity of naming certain butterflies in Colorado and vicinity that are distinctive, but currently have no name, as part of our goal of applying correct species/ subspecies names to all Colorado butterflies. Eleven of those distinctive butterflies are named here, in the genera Anthocharis, Neominois, Asterocampa, Argynnis (Speyeria), Euphydryas, Lycaena, and Hesperia. New life histories are reported for species or subspecies of Neominois & Oeneis & Euphydryas & Lycaena that were recently described or recently elevated in status. Lycaena jlorUs differs in hostplant, egg morphology, and somewhat in a seta on 151 -stage larvae. We also report the results ofresearch elsewhere in North America that was needed to determine which of the current subspecies names should be applied to other butterflies in Colorado, in the genera Anthocharis, Neominois, Apodemia, Callophrys, At/ides, Euphilotes, PlebeJus, Polites, & Hylephila. This research has added additional species to the total of Colorado butterflies. Nomenclatural problems in Colorado Lycaena & Calloph1ys are settled with lectotypes and designations of type localities and two pending petitions to suppress toxotaxa. Difficulties with the ICZN Code in properly applying names to clines are explored, and new terminology is given to some necessary biological solutions. -
![Effects of Canopy Coverage on the Immature Stages of the Clouded Apollo Butterfly [Parnassius Mnemosyne (L.)] with Observations on Larval Behaviour](https://docslib.b-cdn.net/cover/7545/effects-of-canopy-coverage-on-the-immature-stages-of-the-clouded-apollo-butterfly-parnassius-mnemosyne-l-with-observations-on-larval-behaviour-2307545.webp)
Effects of Canopy Coverage on the Immature Stages of the Clouded Apollo Butterfly [Parnassius Mnemosyne (L.)] with Observations on Larval Behaviour
© Entomologica Fennica. 17 June 2005 Effects of canopy coverage on the immature stages of the Clouded Apollo butterfly [Parnassius mnemosyne (L.)] with observations on larval behaviour Panu Välimäki & Juhani Itämies Välimäki, P. & Itämies, J. 2005: Effects of canopy coverage on the immature stages of the Clouded Apollo butterfly [Parnassius mnemosyne (L.)] with obser- vations on larval behaviour. — Entomol. Fennica 16: 117–123. The immature stages of the Clouded Apollo (Parnassius mnemosyne)areas- sumed to be thermophilic due to the possible time limitations and variable weather conditions during their development. Thus, the degree of canopy cover- age may affect habitat use by the species. We explored the spatial distribution of larvae and the development time of pupae under variable canopy coverage condi- tions. Larvae were most abundant in the areas exposed to direct sunlight, al- though the last instar larvae are mobile. Larvae also basked under litter between their foraging periods, probably to enhance digestion and the food intake rate. Moreover, pupal development was retarded by increasing canopy coverage. Pro- longed pupal development and larval avoidance of Corydalis growths under tree canopies indicate that the species suffers from overgrowing and consequently in- creasing canopy coverage. P. Välimäki & J. Itämies, University of Oulu, Department of Zoology, P. O. Box 3000, FI-90014 University of Oulu, Finland; E-mail: [email protected], [email protected] Received 28 May 2003, accepted 13 February 2004 1. Introduction nature conservation act in 1976 [Maa- ja metsä- talousministeriö (1976), see also Mikkola & The Clouded Apollo butterfly [Parnassius Häkkinen (1977)]. However, effective conserva- mnemosyne (L.)] is considered a threatened spe- tion is not possible if the biology of the species is cies in northern and central Europe (Heath 1981, not adequately known. -

Deciphering Range Dynamics
Journal of Biogeography (J. Biogeogr.) (2016) 43, 2186–2198 ORIGINAL Deciphering range dynamics: effects of ARTICLE niche stability areas and post-glacial colonization on alpine species distribution Silvio Marta1*, Federica Lacasella2, Paolo Gratton3, Donatella Cesaroni4 and Valerio Sbordoni4 1Institute of Ecosystem Studies – National ABSTRACT Research Council c/o Department of Biology, Aim Niche stability areas (NSAs) are portions of the species range where climate University of Rome ‘Tor Vergata’, Via della conditions remain suitable through time. They represent the core of species Ricerca Scientifica, 00133 Rome, Italy, 2Department of Entomology, University of ranges. Their distribution and extent, coupled with dispersal and colonization, Wisconsin, 1552 University Avenue – shape the realized range of species. In this study, we quantified the roles of sur- Wisconsin Energy Institute, 53706 Madison, vival within NSAs and post-glacial dispersal in determining the current distribu- WI, USA, 3Department of Primatology, Max tion of two groups of alpine butterflies (two taxa in the Erebia tyndarus species Planck Institute for Evolutionary complex; three taxa in the Parnassius apollo–P. phoebus species complex). Anthropology, Deutscher Platz 6, 04103 Location Holarctic. Leipzig, Germany, 4Department of Biology, University of Rome ‘Tor Vergata’, Via della Methods NSAs were identified for each taxon by combining current and past Ricerca Scientifica, 00133 Rome, Italy potential distributions models, estimated using different modelling techniques and general circulation models. We then (1) assessed the distributional bias towards NSAs by comparing actual occurrence records with randomized occu- pancies of the current potential range and (2) quantified post-glacial dispersal by examining the distribution of distances from each occurrence record to the nearest NSA. -

Pleistocene Evolutionary History of the Clouded Apollo (Parnassius
Molecular Ecology (2008) 17, 4248–4262 doi: 10.1111/j.1365-294X.2008.03901.x PleistoceneBlackwell Publishing Ltd evolutionary history of the Clouded Apollo (Parnassius mnemosyne): genetic signatures of climate cycles and a ‘time-dependent’ mitochondrial substitution rate P. GRATTON,* M. K. KONOPI5SKI† and V. SBORDONI* *Department of Biology, University of Tor Vergata, Via Cracovia 1, 00133 Roma, Italy, †Institute of Nature Conservation PAS, al. Mickiewicza 33, 31–120 Kraków, Poland Abstract Genetic data are currently providing a large amount of new information on past distribution of species and are contributing to a new vision of Pleistocene ice ages. Nonetheless, an increasing number of studies on the ‘time dependency’ of mutation rates suggest that date assessments for evolutionary events of the Pleistocene might be overestimated. We analysed mitochondrial (mt) DNA (COI) sequence variation in 225 Parnassius mnemosyne individuals sampled across central and eastern Europe in order to assess (i) the existence of genetic signatures of Pleistocene climate shifts; and (ii) the timescale of demographic and evolutionary events. Our analyses reveal a phylogeographical pattern markedly influenced by the Pleis- tocene/Holocene climate shifts. Eastern Alpine and Balkan populations display compara- tively high mtDNA diversity, suggesting multiple glacial refugia. On the other hand, three widely distributed and spatially segregated lineages occupy most of northern and eastern Europe, indicating postglacial recolonization from different refugial areas. We show that a conventional ‘phylogenetic’ substitution rate cannot account for the present distribution of genetic variation in this species, and we combine phylogeographical pattern and palaeoeco- logical information in order to determine a suitable intraspecific rate through a Bayesian coalescent approach. -

AC18 Doc. 8.1
AC18 Doc. 8.1 CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ___________________ Eighteenth meeting of the Animals Committee San José (Costa Rica), 8-12 April 2002 Periodic review of animal taxa in the Appendices (Resolution Conf. 11.1) REPORT OF THE WORKING GROUP This document has been prepared by the Chairman of the working group on review of animal taxa in the Appendices. Introduction 1. The North American Regional Representative has been coordinating an intersessional working group on review of animal taxa in the Appendices, with the goal of making significant progress on tasks resulting from deliberations at AC 17. Tasks identified at AC 17 included: (a) the completion of various taxon reviews identified at AC 15 and AC 16; (b) development of guidelines for conducting reviews of animal taxa in the Appendices, (c) development of a rapid assessment technique for screening multiple taxa (or higher-order taxa) at one time; and (d) evaluation of crocodile ranching operations in the framework of the Review of the Appendices. Remaining Species from AC 15 through AC 16 2. At the conclusion of AC17, 11 species reviews remained to be completed, and one preliminary draft review (Cnemidophorus hyperythrus) was identified for revision (Table 1). In the period since AC17, the review of Cnemidophorus hyperythrus has been completed, and two of the other species reviews have been completed (Anas aucklandica, Parnassius apollo). This low completion percentage is one drawback to the voluntary nature of the review process, and lends support to the need to look at alternative approaches for getting reviews done.