Effects of Canopy Coverage on the Immature Stages of the Clouded Apollo Butterfly [Parnassius Mnemosyne (L.)] with Observations on Larval Behaviour
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Monitoring Methodology and Protocols for 20 Habitats, 20 Species and 20 Birds
1 Finnish Environment Institute SYKE, Finland Monitoring methodology and protocols for 20 habitats, 20 species and 20 birds Twinning Project MK 13 IPA EN 02 17 Strengthening the capacities for effective implementation of the acquis in the field of nature protection Report D 3.1. - 1. 7.11.2019 Funded by the European Union The Ministry of Environment and Physical Planning, Department of Nature, Republic of North Macedonia Metsähallitus (Parks and Wildlife Finland), Finland The State Service for Protected Areas (SSPA), Lithuania 2 This project is funded by the European Union This document has been produced with the financial support of the European Union. Its contents are the sole responsibility of the Twinning Project MK 13 IPA EN 02 17 and and do not necessarily reflect the views of the European Union 3 Table of Contents 1. Introduction .......................................................................................................................................................... 6 Summary 6 Overview 8 Establishment of Natura 2000 network and the process of site selection .............................................................. 9 Preparation of reference lists for the species and habitats ..................................................................................... 9 Needs for data .......................................................................................................................................................... 9 Protocols for the monitoring of birds .................................................................................................................... -

Assessing Landscape Effects on Genetics and Dispersal of the Rocky Mountain Apollo Butterfly Arnassiusp Smintheus Using a Resistance Mapping Approach
Western University Scholarship@Western Electronic Thesis and Dissertation Repository 10-27-2017 10:30 AM Assessing Landscape Effects on Genetics and Dispersal of the Rocky Mountain Apollo Butterfly arnassiusP smintheus using a Resistance Mapping Approach Ning Chen The University of Western Ontario Supervisor Dr. Nusha Keyghobadi The University of Western Ontario Graduate Program in Biology A thesis submitted in partial fulfillment of the equirr ements for the degree in Master of Science © Ning Chen 2017 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Biology Commons Recommended Citation Chen, Ning, "Assessing Landscape Effects on Genetics and Dispersal of the Rocky Mountain Apollo Butterfly Parnassius smintheus using a Resistance Mapping Approach" (2017). Electronic Thesis and Dissertation Repository. 5058. https://ir.lib.uwo.ca/etd/5058 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. Abstract Landscape variables that best explain genetic differentiation may not also best explain dispersal patterns, but many studies use genetic differentiation as a proxy for dispersal. I tested the effects of landscape on both genetic differentiation and dispersal in parallel, to explore whether landscape effects on genetic differentiation between populations and landscape effects on dispersal would be comparable in such contexts. I used circuit theory (Circuitscape) and least cost transect analysis to evaluate the effects of landscape on both movement and genetic differentiation of the butterfly, Parnassius smintheus, in the Jumpingpound Ridge study system. -

Introduction
BULGARIA Nick Greatorex-Davies. European Butterflies Group Contact ([email protected]) Local Contact Prof. Stoyan Beshkov. ([email protected]) National Museum of Natural History (NMNH), Sofia, Butterfly Conservation Europe Partner Bulgarian Academy of Sciences Stanislav Abadjiev compiled and collated butterfly records for the whole of Bulgaria and published a Local Recording Scheme distribution atlas in 2001 (see below). Records are still being gathered and can be sent to Stoyan Beshkov at NMNH, Sofia. Butterfly List See Butterflies of Bulgaria website (Details below) Introduction Bulgaria is situated in eastern Europe with its eastern border running along the Black Sea coast. It is separated from Romania for much of its northern border by the River Danube. It shares its western border with Serbia and Macedonia, and its southern border with Greece and Turkey. Bulgaria has a land area of almost 111,000 sq km (smaller than England but bigger than Scotland) and a declining human population of 7.15 million (as of 2015), 1.5 million of which live in the capital city, Sofia. It is very varied in both climate, topography and habitats. Substantial parts of the country are mountainous, particularly in the west, south-west and central ‘spine’ of the country and has the highest mountain in the Balkan Mountains (Musala peak in the Rila Mountains, 2925m) (Map 1). Almost 70% of the land area is above 200m and over 27% above 600m. About 40% of the country is forested and this is likely to increase through natural regeneration due to the abandonment of agricultural land. Following nearly 500 years under the rule of the Ottoman Empire, Bulgaria was independent for just a few years from 1908 before coming under the domination of the soviet communist regime in 1946. -

Anthropogenic Threats to High-Altitude Parnassian Diversity Fabien Condamine, Felix Sperling
Anthropogenic threats to high-altitude parnassian diversity Fabien Condamine, Felix Sperling To cite this version: Fabien Condamine, Felix Sperling. Anthropogenic threats to high-altitude parnassian diversity. News of The Lepidopterists’ Society, 2018, 60, pp.94-99. hal-02323624 HAL Id: hal-02323624 https://hal.archives-ouvertes.fr/hal-02323624 Submitted on 23 Oct 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. _______________________________________________________________________________________News of The Lepidopterists’ Society Volume 60, Number 2 Conservation Matters: Contributions from the Conservation Committee Anthropogenic threats to high-altitude parnassian diversity Fabien L. Condamine1 and Felix A.H. Sperling2 1CNRS, UMR 5554 Institut des Sciences de l’Evolution (Université de Montpellier), Place Eugène Bataillon, 34095 Montpellier, France [email protected], corresponding author 2Department of Biological Sciences, University of Alberta, Edmonton T6G 2E9, Alberta, Canada Introduction extents (Chen et al. 2011). However, this did not result in increases in range area because the area of land available Global mean annual temperatures increased by ~0.85° declines with increasing elevation. Accordingly, extinc- C between 1880 and 2012 and are likely to rise by an tion risk may increase long before species reach a summit, additional 1° C to 4° C by 2100 (Stocker et al. -
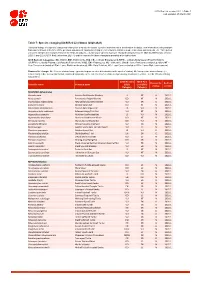
Table 7: Species Changing IUCN Red List Status (2020-2021)
IUCN Red List version 2021-1: Table 7 Last Updated: 25 March 2021 Table 7: Species changing IUCN Red List Status (2020-2021) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2020 (IUCN Red List version 2020-3) and 2021 (IUCN Red List version 2021-1) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2020) List (2021) change version Category -

Habitat Utilization and Behaviour of Adult Parnassius Mnemosyne (Lepidoptera: Papilionidae) in the Litovelské Pomoravi, Czech Republic
©Societas Europaea Lepidopterologica; download unter http://www.biodiversitylibrary.org/ und www.zobodat.at Nota lepid. 24 (1/2): 39-51 39 Habitat utilization and behaviour of adult Parnassius mnemosyne (Lepidoptera: Papilionidae) in the Litovelské Pomoravi, Czech Republic Martin Konvicka*, Martin Duchoslav**, Milena Harastovà* ", Jiri Benes**, sllvie foldynovà**, mllos jlrkû** & tomas kuras** Faculty of Biological Sciences, University of South Bohemia, Branisovskâ 31, Ceské Budëjovice, CZ-370 05, Czech Republic. E-mail: [email protected] Faculty of Sciences, Palacky University, Svobody 26, Olomouc, CZ-771 41, Czech Republic ** Faculty of Agriculture, University of South Bohemia, Studentskâ 13, Ceské Budëjovice, CZ-370 05, Czech Republic Summary. Within-habitat distribution and diurnal behaviour of adults of the Clouded Apollo (Parnassius mmemosyne) were studied in the Litovelské Pomoravi, Moravia, Czech Republic. Data were collected by censuses along a regular transect route, which crossed three clearings in a mature deciduous forest. A loglinear model was constructed in order to explain variability in collected behavioural data. Hour of observation, Transect section, Behaviour, Sex and the interactions among factors Transect section-Be- haviour and Sex-Behaviour significantly influenced the number of butterflies seen. More males com- pared to females were seen, both sexes markedly prevailed at the clearings, they hardly ever entered the high forest. Mating, oviposition and late afternoon basking-resting were all also restricted to the clear- ings. These findings further highlight the importance of sunny forest gaps for conservation of the species in Central Europe. There was no distinct diurnal pattern in behaviour, except for the fact that males patrolled for most of day-time, but predominantly basked in early mornings and late afternoons. -

Pleistocene Evolutionary History of the Clouded Apollo (Parnassius
Molecular Ecology (2008) 17, 4248–4262 doi: 10.1111/j.1365-294X.2008.03901.x PleistoceneBlackwell Publishing Ltd evolutionary history of the Clouded Apollo (Parnassius mnemosyne): genetic signatures of climate cycles and a ‘time-dependent’ mitochondrial substitution rate P. GRATTON,* M. K. KONOPI5SKI† and V. SBORDONI* *Department of Biology, University of Tor Vergata, Via Cracovia 1, 00133 Roma, Italy, †Institute of Nature Conservation PAS, al. Mickiewicza 33, 31–120 Kraków, Poland Abstract Genetic data are currently providing a large amount of new information on past distribution of species and are contributing to a new vision of Pleistocene ice ages. Nonetheless, an increasing number of studies on the ‘time dependency’ of mutation rates suggest that date assessments for evolutionary events of the Pleistocene might be overestimated. We analysed mitochondrial (mt) DNA (COI) sequence variation in 225 Parnassius mnemosyne individuals sampled across central and eastern Europe in order to assess (i) the existence of genetic signatures of Pleistocene climate shifts; and (ii) the timescale of demographic and evolutionary events. Our analyses reveal a phylogeographical pattern markedly influenced by the Pleis- tocene/Holocene climate shifts. Eastern Alpine and Balkan populations display compara- tively high mtDNA diversity, suggesting multiple glacial refugia. On the other hand, three widely distributed and spatially segregated lineages occupy most of northern and eastern Europe, indicating postglacial recolonization from different refugial areas. We show that a conventional ‘phylogenetic’ substitution rate cannot account for the present distribution of genetic variation in this species, and we combine phylogeographical pattern and palaeoeco- logical information in order to determine a suitable intraspecific rate through a Bayesian coalescent approach. -

Management Plan for Virgin Komi Forests UNESCO Site; First of All, They Are Employees of Yugyd Va National Park and Pechora-Ilych State Nature Reserve Fsbis
Ministry of Natural Resources and Environment of the Russian Federation Yugyd Va National Park Federal State Budgetary Institution Pechora-Ilych State Nature Reserve Federal State Budgetary Institution "Republican Center for Ensuring the Functioning of Specially Protected Natural Territories and Nature Management" State Budgetary Institution of the Republic of Komi of the Ministry of Industry, Natural Resources, Energy and Transport of the Republic of Komi MANAGEMENT PLAN for Virgin Komi Forests UNESCO World Natural Heritage Site 2017 – 2031 Vuktyl-Yaksha-Syktyvkar 2017 1 TABLE OF CONTENTS SUMMARY 5 INTRODUCTION 8 1. General Information on the Site 10 2. Natural and Historical and Cultural Values of the Site 15 3. Social and Economic Conditions and Nature Management 30 4. Site Management 35 5. Action Plan - General Provisions 56 5.1. Targeted Action Plan Programs 63 5.1.1. Program for the conservation of natural complexes in natural state 63 5.1.2. Program for detection and suppression of violations of environment-oriented 69 legislation 5.1.3. Program for environmental education 74 5.1.4. Program for scientific research works and environmental monitoring 81 5.1.5. Program for conservation and restoration of complexes and sites 83 5.1.6. Program for landscaping works for the development of regulated tourism, 85 recreation and environmental education activities 5.1.7. Administration and financial and economic activities 89 Estimation of financial costs for implementation of the management plan 91 6. Monitoring the implementation of the management plan 92 Complex monitoring. Indicators for the implementation of the management plan. 7. Operational plan for the first year implementation 105 Financing gap for the programs of the Site APPENDICES Appendix 1. -

AC18 Doc. 8.1
AC18 Doc. 8.1 CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ___________________ Eighteenth meeting of the Animals Committee San José (Costa Rica), 8-12 April 2002 Periodic review of animal taxa in the Appendices (Resolution Conf. 11.1) REPORT OF THE WORKING GROUP This document has been prepared by the Chairman of the working group on review of animal taxa in the Appendices. Introduction 1. The North American Regional Representative has been coordinating an intersessional working group on review of animal taxa in the Appendices, with the goal of making significant progress on tasks resulting from deliberations at AC 17. Tasks identified at AC 17 included: (a) the completion of various taxon reviews identified at AC 15 and AC 16; (b) development of guidelines for conducting reviews of animal taxa in the Appendices, (c) development of a rapid assessment technique for screening multiple taxa (or higher-order taxa) at one time; and (d) evaluation of crocodile ranching operations in the framework of the Review of the Appendices. Remaining Species from AC 15 through AC 16 2. At the conclusion of AC17, 11 species reviews remained to be completed, and one preliminary draft review (Cnemidophorus hyperythrus) was identified for revision (Table 1). In the period since AC17, the review of Cnemidophorus hyperythrus has been completed, and two of the other species reviews have been completed (Anas aucklandica, Parnassius apollo). This low completion percentage is one drawback to the voluntary nature of the review process, and lends support to the need to look at alternative approaches for getting reviews done. -

Translocation of the Endangered Apollo Butterfly Parnassius Apollo in Southern Finland
M.S. Fred & J.E. Brommer / Conservation Evidence (2015) 12, 8-13 Translocation of the endangered apollo butterfly Parnassius apollo in southern Finland Marianne S. Fred1 & Jon E. Brommer2* 1 Aronia Research and Development Institute, Novia University of Applied Sciences, Finland 2 Department of Biology, University of Turku, Finland. SUMMARY Translocation of individuals across a barrier which hampers natural colonisation is a potentially important, but debated, conservation tool for a variety of organisms in a world altered by anthropogenic influences. The apollo Parnassius apollo is an endangered butterfly whose distribution retracted dramatically during the 1900s across Europe. In Finland the apollo currently occupies only a fraction of the range of its suitable habitat and is apparently unable to re-colonise other areas. Using eggs collected from wild-caught females from the species’ current Finnish stronghold, a population was reared in order to translocate larvae into an unoccupied, but highly suitable, part of the Finnish archipelago where the species historically occurred until its national decline in the 1950s. In 2009 a restricted number of larvae (1 larva/10 host plants) were released on 25 islands in the inner, middle and outer archipelago zones. In 2010, nine islands situated in all three archipelago zones were (re)stocked with a high density of larvae (1/host plant). In 2011, apollo larval populations were found only on islands in the outer archipelago zone, which were then restocked. The species remained present here in the following two years (2012, 2013) and was hence able to sustain multi-annual population establishment without restocking. Our findings demonstrate that empty suitable habitat may in reality consist of only a few sites where population establishment is possible. -

The I5k Initiative: Advancing Arthropod Genomics for Knowledge
University of Rhode Island DigitalCommons@URI Biological Sciences Faculty Publications Biological Sciences 2013 The i5K nitI iative: Advancing Arthropod Genomics for Knowledge, Human Health, Agriculture, and the Environment i5K Consortium Marian Goldsmith University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/bio_facpubs The University of Rhode Island Faculty have made this article openly available. Please let us know how Open Access to this research benefits oy u. This is a pre-publication author manuscript of the final, published article. Terms of Use This article is made available under the terms and conditions applicable towards Open Access Policy Articles, as set forth in our Terms of Use. Citation/Publisher Attribution Goldsmith, M. (2013). The i5KI nitI iative: Advancing Arthropod Genomics for Knowledge, Human Health, Agriculture, and the Environment. Journal of Heredity, 104(5), 595–600. Available at: http://dx.doi.org/10.1093/jhered/est050 This Article is brought to you for free and open access by the Biological Sciences at DigitalCommons@URI. It has been accepted for inclusion in Biological Sciences Faculty Publications by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. Journal of Heredity 2013:104(5):595–600 Published by Oxford University Press on behalf of The American Genetic doi:10.1093/jhered/est050 Association 2013. This work is written by (a) US Government employee(s) and is in the public domain in the US The i5K Initiative: Advancing Arthropod Genomics for Knowledge, Human Health, Agriculture, and the Environment I5K CONSORTIUM* Downloaded from *Authors listed in the Appendix. -
Ref: CL/WHC/14/02 27 November 2002 To
United Nations Educational, Scientific and Cultural Organization Organisation des Nations Unies pour l'éducation, la science et la culture 7, place de Fontenoy 75352 Paris 07 SP Tel. : + 33 (0) 1.45.68.15.71 Fax : + 33 (0) 1.45.68.55.70 Ref: CL/WHC/14/02 27 November 2002 To: Permanent Delegations, Observer Missions and National Commissions of States Parties to the UNESCO World Heritage Convention in Europe and North America Madam/Sir, Subject: Periodic Reporting on the application of the World Heritage Convention and on the state of conservation of World Heritage properties in Europe and North America I have the honor to draw your attention to Article 29 of the 1972 UNESCO World Heritage Convention which requests that “The States Parties to this Convention shall, in the reports which they submit to the General Conference of the United Nations Educational, Scientific and Cultural Organization (…), give information on the legislative and administrative provisions which they have adopted and other action which they have taken for the application of this Convention, together with details of the experience acquired in this field”. The twenty-ninth General Conference of UNESCO in 1997, requested the World Heritage Committee to define the periodicity, form, nature and extent of the periodic reporting on the application of the World Heritage Convention and on the state of conservation of World Heritage properties and to examine and respond to these reports. The World Heritage Committee, at its twenty-second session in December 1998, decided to invite States Parties to the World Heritage Convention to submit the periodic reports every six years in accordance with the Format for periodic reports as adopted by the Committee.