United States Patent Office
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Proton Nmr Spectroscopy
1H NMR Spectroscopy (#1c) The technique of 1H NMR spectroscopy is central to organic chemistry and other fields involving analysis of organic chemicals, such as forensics and environmental science. It is based on the same principle as magnetic resonance imaging (MRI). This laboratory exercise reviews the principles of interpreting 1H NMR spectra that you should be learning right now in Chemistry 302. There are four questions you should ask when you are trying to interpret an NMR spectrum. Each of these will be discussed in detail. The Four Questions to Ask While Interpreting Spectra 1. How many different environments are there? The number of peaks or resonances (signals) in the spectrum indicates the number of nonequivalent protons in a molecule. Chemically equivalent protons (magnetically equivalent protons) give the same signal in the NMR whereas nonequivalent protons give different signals. 1 For example, the compounds CH3CH3 and BrCH2CH2Br all have one peak in their H NMR spectra because all of the protons in each molecule are equivalent. The compound below, 1,2- dibromo-2-methylpropane, has two peaks: one at 1.87 ppm (the equivalent CH3’s) and the other at 3.86 ppm (the CH2). 1.87 1.87 ppm CH 3 3.86 ppm Br Br CH3 1.87 ppm 3.86 10 9 8 7 6 5 4 3 2 1 0 2. How many 1H are in each environment? The relative intensities of the signals indicate the numbers of protons that are responsible for individual signals. The area under each peak is measured in the form of an integral line. -
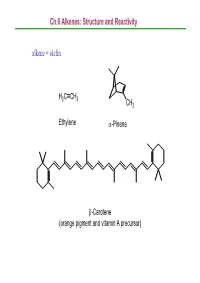
Ch.6 Alkenes: Structure and Reactivity Alkene = Olefin
Ch.6 Alkenes: Structure and Reactivity alkene = olefin H2CCH2 CH3 Ethylene α-Pinene β-Carotene (orange pigment and vitamin A precursor) Ch.6 Alkenes: Structure and Reactivity 6.1 Industrial Preparation and Use of Alkenes Compounds derived industrially from ethylene CH3CH2OH Ethanol CH3CHO Acetaldehyde CH3COOH Acetic acid HOCH2CH2OH Ethylene glycol ClCH2CH2Cl Ethylene dichloride H C=CHCl Vinyl chloride H2CCH2 2 O Ethylene oxide Ethylene (26 million tons / yr) O Vinyl acetate O Polyethylene Ch.6 Alkenes: Structure and Reactivity Compounds derived industrially from propylene OH Isopropyl alcohol H3CCH3 O Propylene oxide CH3 H3CCH CH2 Propylene Cumene (14 million tons / yr) CH3 CH3 Polypropylene Ch.6 Alkenes: Structure and Reactivity • Ethylene, propylene, and butene are synthesized industrially by thermal cracking of natural gas (C1-C4 alkanes) and straight-run gasoline (C4-C8 alkanes). 850-900oC CH (CH ) CH H + CH + H C=CH + CH CH=CH 3 2 n 3 steam 2 4 2 2 3 2 + CH3CH2CH=CH2 - the exact processes are complex; involve radical process H 900oC CH3CH2 CH2CH3 22H2CCH H2C=CH2 +H2 Ch.6 Alkenes: Structure and Reactivity • Thermal cracking is an example of a reaction whose energetics are dominated by entropy (∆So) rather than enthalpy (∆Ho) in the free-energy equation (∆Go = ∆Ho -T∆So) . ; C-C bond cleavage (positive ∆Ho) ; high T and increased number of molecules → larger T∆So Ch.6 Alkenes: Structure and Reactivity 6.2 Calculating Degree of Unsaturation unsaturated: formula of alkene CnH2n ; formula of alkane CnH2n+2 in general, each ring or double -

Ganic Compounds
6-1 SECTION 6 NOMENCLATURE AND STRUCTURE OF ORGANIC COMPOUNDS Many organic compounds have common names which have arisen historically, or have been given to them when the compound has been isolated from a natural product or first synthesised. As there are so many organic compounds chemists have developed rules for naming a compound systematically, so that it structure can be deduced from its name. This section introduces this systematic nomenclature, and the ways the structure of organic compounds can be depicted more simply than by full Lewis structures. The language is based on Latin, Greek and German in addition to English, so a classical education is beneficial for chemists! Greek and Latin prefixes play an important role in nomenclature: Greek Latin ½ hemi semi 1 mono uni 1½ sesqui 2 di bi 3 tri ter 4 tetra quadri 5 penta quinque 6 hexa sexi 7 hepta septi 8 octa octo 9 ennea nona 10 deca deci Organic compounds: Compounds containing the element carbon [e.g. methane, butanol]. (CO, CO2 and carbonates are classified as inorganic.) See page 1-4. Special characteristics of many organic compounds are chains or rings of carbon atoms bonded together, which provides the basis for naming, and the presence of many carbon- hydrogen bonds. The valency of carbon in organic compounds is 4. Hydrocarbons: Compounds containing only the elements C and H. Straight chain hydrocarbons are named according to the number of carbon atoms: CH4, methane; C2H6 or H3C-CH3, ethane; C3H8 or H3C-CH2-CH3, propane; C4H10 or H3C-CH2- CH2-CH3, butane; C5H12 or CH3CH2CH2CH2CH3, pentane; C6H14 or CH3(CH2)4CH3, hexane; C7H16, heptane; C8H18, octane; C9H20, nonane; C10H22, CH3(CH2)8CH3, decane. -

Surface Chemistry Changes of Weathered HDPE/Wood-Flour
Polymer Degradation and Stability 86 (2004) 1–9 www.elsevier.com/locate/polydegstab Surface chemistry changes of weathered HDPE/wood-flour composites studied by XPS and FTIR spectroscopy* Nicole M. Starka,), Laurent M. Matuanab aU.S. Department of Agriculture, Forest Service, Forest Products Laboratory, One Gifford Pinchot Drive, Madison, WI 53726-2398, United States bDepartment of Forestry, Michigan State University, East Lansing, MI 48824-1222, United States Received 7 August 2003; received in revised form 3 November 2003; accepted 4 November 2003 Abstract The use of wood-derived fillers by the thermoplastic industry has been growing, fueled in part by the use of wood-fiber– thermoplastic composites by the construction industry. As a result, the durability of wood-fiber–thermoplastic composites after ultraviolet exposure has become a concern. Samples of 100% high-density polyethylene (HDPE) and HDPE filled with 50% wood- flour (WF) were weathered in a xenon arc-type accelerated weathering apparatus for 2000 h. Changes in surface chemistry were studied using spectroscopic techniques. X-ray photoelectron spectroscopy (XPS) was used to verify the occurrence of surface oxidation. Fourier transform infrared (FTIR) spectroscopy was used to monitor the development of degradation products, such as carbonyl groups and vinyl groups, and to determine changes in HDPE crystallinity. The results indicate that surface oxidation occurred immediately after exposure for both the neat HDPE and WF/HDPE composites; the surface of the WF/HDPE composites was oxidized to a greater extent than that of the neat HDPE. This suggests that the addition of WF to the HDPE matrix results in more weather-related damage. -

Novel Poly(Vinyl Alcohol)-Based Column Coating for Capillary Electrophoresis of Proteins
Biochemical Engineering Journal 53 (2010) 137–142 Contents lists available at ScienceDirect Biochemical Engineering Journal journal homepage: www.elsevier.com/locate/bej Novel poly(vinyl alcohol)-based column coating for capillary electrophoresis of proteins Liang Xu, Xiao-Yan Dong, Yan Sun ∗ Department of Biochemical Engineering and Key Laboratory of Systems Bioengineering of the Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China article info abstract Article history: A novel and simple method for the preparation of chemically bonded poly(vinyl alcohol) (PVA) coat- Received 30 June 2010 ing to silica capillary inner wall was developed, and the PVA-coated capillary columns were employed Received in revised form for capillary electrophoresis (CE). The coating procedure included pretreatment of the capillary inner 30 September 2010 wall, silanization, aldehyde group functionalization and PVA immobilization. Electroosmotic flow of the Accepted 6 October 2010 coated capillary was almost suppressed over a wide pH range (pH 3–10). High-efficiency separations of cationic proteins (including cytochrome c, lysozyme, ␣-chymotrypsinogen A) at pH 3.0–5.0 and of anionic proteins (including myoglobin and trypsin inhibitor) at pH 10.0 were achieved with the PVA-coated cap- Keywords: Protein illary. Moreover, a “dual-opposite-injection” approach was adopted for simultaneous separations of both Separation cationic and anionic proteins at neutral pH with the prepared column. In this CE mode, positively charged Bioprocess Monitoring proteins migrated from one end of the column to the detector while negatively charged proteins from Adsorption the other end to the detection window. Good run-to-run repeatability was obtained in all of the protein Capillary electrophoresis CE separations performed in this work. -

Alkynes: Molecular and Structural Formulas
Alkynes: Molecular and Structural Formulas The alkynes comprise a series of carbon- and hydrogen-based compounds that contain at least one triple bond. This group of compounds is a homologous series with the general molecular formula of C n H2 n--2 , where n equals any integer greater than one. The simplest alkyne, ethyne (also known as acetylene), has two carbon atoms and the molecular formula of C2H2. The structural formula for ethyne is In longer alkyne chains, the additional carbon atoms are attached to each other by single covalent bonds. Each carbon atom is also attached to sufficient hydrogen atoms to produce a total of four single covalent bonds about itself. In alkynes of four or more carbon atoms, the triple bond can be located in different positions along the chain, leading to the formation of structural isomers. For example, the alkyne of molecular formula C4H6 has two isomers, Although alkynes possess restricted rotation due to the triple bond, they do not have stereoisomers like the alkenes because the bonding in a carbon-carbon triple bond is sp hybridized. In sp hybridization, the maximum separation between the hybridized orbitals is 180°, so the molecule is linear. Thus, the substituents on triple-bonded carbons are positioned in a straight line, and stereoisomers are impossible Alkynes: Nomenclature Although some common alkyne names, such as acetylene, are still found in many textbooks, the International Union of Pure and Applied Chemistry (IUPAC) nomenclature is required for journal articles. The rules for alkynes in this system are identical with those for alkenes, except for the ending. -

1-Alkenylboranes And(E)-1-Alkenylboronates an Investigation of Borane/Nitrone Reactions
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 1991 Preparation and Characterization of (E)-1-Alkenylboranes and(E)-1-Alkenylboronates an Investigation of Borane/Nitrone Reactions Philip Louis Smith College of William & Mary - Arts & Sciences Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the Organic Chemistry Commons Recommended Citation Smith, Philip Louis, "Preparation and Characterization of (E)-1-Alkenylboranes and(E)-1-Alkenylboronates an Investigation of Borane/Nitrone Reactions" (1991). Dissertations, Theses, and Masters Projects. Paper 1539625667. https://dx.doi.org/doi:10.21220/s2-902s-jv27 This Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. Preparation and characterization of (E)-l-Alkenylboranes and (E)-l-Alkenylboronates And An Investigation of Borane/Nitrone Reactions A Thesis Presented to The Faculty of the Department of Chemistry The College of William and Mary in Virginia In Partial Fulfillment Of the Requirements for the Degree of Masters of Arts by Philip L. Smith 1991 APPROVAL SHEET This thesis is submitted in partial fulfillment of the requirements for the degree of Masters of Arts L Philip L. Smith Approved, September 1991 + W. Gary Hollis, Ph/D. toi/M 6/. David W. ThompsofvPh. D Christopher J. AbeW, Ph.D. DEDICATION For George and Herminia, my parents, Stella and Kimberly, my sisters, and Christain, my brother. iii ACKNOWLEDGMENTS I wish to express my sincere appreciation to Dr. -
Organic Structures 2
Organic structures 2 Connections Building on Arriving at Looking forward to • This chapter does not depend on • The diagrams used in the rest of the book • Ascertaining molecular structure Chapter 1 • Why we use these particular diagrams spectroscopically ch3 • How organic chemists name molecules • What determines a molecule’s in writing and in speech structure ch4 • What is the skeleton of an organic molecule • What is a functional group • Some abbreviations used by all organic chemists • Drawing organic molecules realistically in an easily understood style There are over 100 elements in the periodic table. Many molecules contain well over 100 ■ Palytoxin was isolated in atoms—palytoxin (a naturally occurring compound with potential anticancer activity), for 1971 in Hawaii from Limu make example, contains 129 carbon atoms, 221 hydrogen atoms, 54 oxygen atoms, and 3 nitrogen o Hane (‘deadly seaweed of atoms. It’s easy to see how chemical structures can display enormous variety, providing Hana’), which had been used to enough molecules to build even the most complicated living creatures. poison spear points. It is one of the most toxic compounds OH known, requiring only about HO OH 0.15 micrograms per kilogram OH OH OH for death by injection. The HO complicated structure was O OH H determined a few years later. HO OH OH O H H O O HO HO H OH OH HO OH OH H H H H O N N OH HO HO H HO OH OH O O O HO OH H OH HO OH NH2 HO OH HO palytoxin HO OH O H HO H OH O H H H HO HO OH O O OH OH HO H Online support. -

Hybrid Copolymerization of Ethylene Oxide and Tert-Butyl Methacrylate with Organocatalyst
polymers Article Hybrid Copolymerization of Ethylene Oxide and tert-Butyl Methacrylate with Organocatalyst Wenhao Xiao 1,†, Liguo Xu 2,†, Pan Liu 1, Yang Chen 1, Jie Zhang 1 and Jinbao Xu 1,* 1 Guangdong Provincial Key Laboratory of Functional Soft Condensed Matter, School of Materials and Energy, Guangdong University of Technology, Guangzhou 510006, China; [email protected] (W.X.); [email protected] (P.L.); [email protected] (Y.C.); [email protected] (J.Z.) 2 School of Materials Science and Engineering, South China University of Technology, Guangzhou 510640, China; [email protected] * Correspondence: [email protected] † These authors contributed equally to this work. Abstract: Hybrid copolymerization of structurally different, reactivity and mechanism distinct monomers (e.g., cyclic and vinyl type monomers) is of great interest and challenge for both academic research and practical application. Herein, ethylene oxide-co-tert-butyl methacrylate-co-poly(ethylene glycol) benzyl methacrylate (EO-co-BMA-co-bPEO), a statistical copolymer was synthesized via hybrid copolymerization of EO and BMA using an uncharged, non-nucleophilic organobase t-BuP4 as the catalyst. Detailed characterizations indicate that hybrid copolymerization of ethylene oxide and vinyl monomer forms a statistical copolymer concurrently with the transesterification of tert- butyl group and oligomer PEO anions. The application of the copolymer as all solid lithium-ion battery polymer electrolyte was investigated by detecting the ionic conductivity (σ) with electrical impedance spectrum measurement. Citation: Xiao, W.; Xu, L.; Liu, P.; Chen, Y.; Zhang, J.; Xu, J. Hybrid Keywords: ethylene oxide; tert-butyl methacrylate; hybrid copolymerization; solid polymer electrolyte Copolymerization of Ethylene Oxide and tert-Butyl Methacrylate with Organocatalyst. -

Glycolurils Having Functional Group and Use Thereof
(19) TZZ¥Z¥_T (11) EP 3 075 735 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: 05.10.2016 Bulletin 2016/40 C07D 487/04 (2006.01) C08G 77/388 (2006.01) C08L 83/05 (2006.01) C08L 83/07 (2006.01) (21) Application number: 14864012.1 (86) International application number: (22) Date of filing: 25.11.2014 PCT/JP2014/081009 (87) International publication number: WO 2015/076399 (28.05.2015 Gazette 2015/21) (84) Designated Contracting States: • TAKEDA, Takuma AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Ayauta-gun GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Kagawa 769-0202 (JP) PL PT RO RS SE SI SK SM TR • MIURA, Syozo Designated Extension States: Ayauta-gun BA ME Kagawa 769-0202 (JP) • KASHIWABARA, Takashi (30) Priority: 25.11.2013 JP 2013242452 Ayauta-gun 26.11.2013 JP 2013243804 Kagawa 769-0202 (JP) 27.11.2013 JP 2013245468 • MIZOBE, Noboru 04.12.2013 JP 2013251166 Ayauta-gun 06.12.2013 JP 2013252748 Kagawa 769-0202 (JP) 27.10.2014 JP 2014218061 (74) Representative: Duckworth, Timothy John (71) Applicant: Shikoku Chemicals Corporation J A Kemp Marugame-shi, Kagawa 763-8504 (JP) 14 South Square Gray’s Inn (72) Inventors: London WC1R 5JJ (GB) • KUMANO, Takeshi Ayauta-gun Kagawa 769-0202 (JP) (54) GLYCOLURILS HAVING FUNCTIONAL GROUP AND USE THEREOF (57) The invention provides a glycoluril represented sents a hydrogen atom, a lower alkyl or a phenyl group; by the general formula (Z): R3, R 4 and R 5each independently representsa hydrogen atom or the group identical with the group Z; however, when the group Z is a carboxyalkyl group, R 3, R4 and R5 are the same carboxyalkyl groups as the group Z; and when the group Z is an allyl group, R5 represents a hy- drogen atom. -

Synthesis of 3D-PEPS According to 2 Related Pathways
Laboratory of Macromolecular And Physical Organic Chemistry Department of Chemistry Katholieke Universiteit Leuven Celestijnenlaan, 200F B-3001 Leuven Belgium Surface Protection Coatings Against Corrosive Materials. Synthesis of 3D-PEPS (Three Dimensional Polyester-Polystyrene) According to 2 related pathways. Introduction In the confusing jungle of commercial nomenclature of polymer chemicals and reactions, a scientific proof is given that 3D-PEPS (three dimentional polyester-polystyrene) copolymer matrix materials are synthesed according to 2 pathways which start from the same classes of raw materials and use the same reaction mechanisms. The intermediate prepolymers of both pathways belong to the same family of reactive prepolymers. More specific: Raws materials classes: Carboxyl acids and diols Reaction mechanisms: Condensation; for the creation of prepolymers Free radical vinyl copolymerisation; crosslinking of prepolymers with vinyl monomers (eg styrene) to form 3D-PEPS matrix. Intermediate prepolymers: DVDE (divinyl-diester) and MVME (multivinyl-multiester) are subfamilies of the PVPE (polyvinyl-polyester) family. Definitions 3D-PEPS = three dimentional polyester-polystyrene: copolymer matrix with multiple ester groups and multiple styrene groups. Commercial names: (crosslinked) polyester PVPE = polyvinyl-polyester : prepolymer with 2 or more vinyl groups and 2 or more ester groups. Commercial names: “unsaturated polyester and vinyl ester”. DVDE = divinyl-diester: prepolymer with 2 vinyl groups and 2 ester groups. The vinyl groups are at the ends of the prepolymer, the ester groups next to them. Commercial names: vinyl ester, unsaturated epoxy, epoxy vinyl ester, phenacrylate, unsaturated monocarboxyl-epoxypolymer condensate, unsaturated monocarboxyl-diol condensate. MVME = multivinyl-multiester: prepolymer with multi (>2) vinyl and multi (>2) ester groups. The vinyl groups and ester groups are located inside the prepolymer. -

4.2 Nomenclature of Alkenes 131
04_BRCLoudon_pgs4-6.qxd 11/26/08 8:39 AM Page 131 4.2 NOMENCLATURE OF ALKENES 131 PROBLEM 4.1 Which of the following alkenes can exist as double-bond stereo? Identify the stereocenters in each. (a) H2CA CHCH2CH2CH3 (b) CH3CH2CHA CHCH2CH3 1-pentene 3-hexene B (c) H2CA CHCH A CH CH3 (d) CH3CH2CHA CCH3 (e) L L 1,3-pentadiene "CH3 cyclobutene 2-methyl-2-pentene (Hint for part (e): Try to build a model of both stereoisomers, but don’t break your models!) 4.2 NOMENCLATURE OF ALKENES A. IUPAC Substitutive Nomenclature The IUPAC substitutive nomenclature of alkenes is derived by modifying alkane nomencla- ture in a simple way. An unbranched alkene is named by replacing the ane suffix in the name of the corresponding alkane with the ending ene and specifying the location of the double bond with a number. The carbons are numbered from one end of the chain to the other so that the double bond receives the lowest number. The carbons of the double bond are numbered consecutively. 12 3456 H2CA CH CH2CH2CH2CH3 1-hexene L hexane ene hexene + = position of double bond The IUPAC recognizes an exception to this rule for the name of the simplest alkene, H2CACH2, which is usually called ethylene rather than ethene. (Chemical Abstracts (Sec. 2.4D, p. 66), however, uses the substitutive name ethene.) The names of alkenes with branched chains are, like those of alkanes, derived from their principal chains. In an alkene, the principal chain is defined as the carbon chain containing the greatest number of double bonds, even if this is not the longest chain.