Alkynes: Molecular and Structural Formulas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Proton Nmr Spectroscopy
1H NMR Spectroscopy (#1c) The technique of 1H NMR spectroscopy is central to organic chemistry and other fields involving analysis of organic chemicals, such as forensics and environmental science. It is based on the same principle as magnetic resonance imaging (MRI). This laboratory exercise reviews the principles of interpreting 1H NMR spectra that you should be learning right now in Chemistry 302. There are four questions you should ask when you are trying to interpret an NMR spectrum. Each of these will be discussed in detail. The Four Questions to Ask While Interpreting Spectra 1. How many different environments are there? The number of peaks or resonances (signals) in the spectrum indicates the number of nonequivalent protons in a molecule. Chemically equivalent protons (magnetically equivalent protons) give the same signal in the NMR whereas nonequivalent protons give different signals. 1 For example, the compounds CH3CH3 and BrCH2CH2Br all have one peak in their H NMR spectra because all of the protons in each molecule are equivalent. The compound below, 1,2- dibromo-2-methylpropane, has two peaks: one at 1.87 ppm (the equivalent CH3’s) and the other at 3.86 ppm (the CH2). 1.87 1.87 ppm CH 3 3.86 ppm Br Br CH3 1.87 ppm 3.86 10 9 8 7 6 5 4 3 2 1 0 2. How many 1H are in each environment? The relative intensities of the signals indicate the numbers of protons that are responsible for individual signals. The area under each peak is measured in the form of an integral line. -
Ch.6 Alkenes: Structure and Reactivity Alkene = Olefin
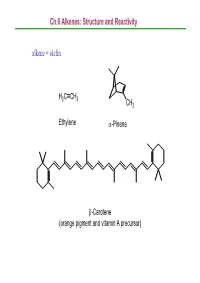
Ch.6 Alkenes: Structure and Reactivity alkene = olefin H2CCH2 CH3 Ethylene α-Pinene β-Carotene (orange pigment and vitamin A precursor) Ch.6 Alkenes: Structure and Reactivity 6.1 Industrial Preparation and Use of Alkenes Compounds derived industrially from ethylene CH3CH2OH Ethanol CH3CHO Acetaldehyde CH3COOH Acetic acid HOCH2CH2OH Ethylene glycol ClCH2CH2Cl Ethylene dichloride H C=CHCl Vinyl chloride H2CCH2 2 O Ethylene oxide Ethylene (26 million tons / yr) O Vinyl acetate O Polyethylene Ch.6 Alkenes: Structure and Reactivity Compounds derived industrially from propylene OH Isopropyl alcohol H3CCH3 O Propylene oxide CH3 H3CCH CH2 Propylene Cumene (14 million tons / yr) CH3 CH3 Polypropylene Ch.6 Alkenes: Structure and Reactivity • Ethylene, propylene, and butene are synthesized industrially by thermal cracking of natural gas (C1-C4 alkanes) and straight-run gasoline (C4-C8 alkanes). 850-900oC CH (CH ) CH H + CH + H C=CH + CH CH=CH 3 2 n 3 steam 2 4 2 2 3 2 + CH3CH2CH=CH2 - the exact processes are complex; involve radical process H 900oC CH3CH2 CH2CH3 22H2CCH H2C=CH2 +H2 Ch.6 Alkenes: Structure and Reactivity • Thermal cracking is an example of a reaction whose energetics are dominated by entropy (∆So) rather than enthalpy (∆Ho) in the free-energy equation (∆Go = ∆Ho -T∆So) . ; C-C bond cleavage (positive ∆Ho) ; high T and increased number of molecules → larger T∆So Ch.6 Alkenes: Structure and Reactivity 6.2 Calculating Degree of Unsaturation unsaturated: formula of alkene CnH2n ; formula of alkane CnH2n+2 in general, each ring or double -

Alkenes and Alkynes
02/21/2019 CHAPTER FOUR Alkenes and Alkynes H N O I Cl C O C O Cl F3C C Cl C Cl Efavirenz Haloprogin (antiviral, AIDS therapeutic) (antifungal, antiseptic) Chapter 4 Table of Content * Unsaturated Hydrocarbons * Introduction and hybridization * Alkenes and Alkynes * Benzene and Phenyl groups * Structure of Alkenes, cis‐trans Isomerism * Nomenclature of Alkenes and Alkynes * Configuration cis/trans, and cis/trans Isomerism * Configuration E/Z * Physical Properties of Hydrocarbons * Acid‐Base Reactions of Hydrocarbons * pka and Hybridizations 1 02/21/2019 Unsaturated Hydrocarbons • Unsaturated Hydrocarbon: A hydrocarbon that contains one or more carbon‐carbon double or triple bonds or benzene‐like rings. – Alkene: contains a carbon‐carbon double bond and has the general formula CnH2n. – Alkyne: contains a carbon‐carbon triple bond and has the general formula CnH2n‐2. Introduction Alkenes ● Hydrocarbons containing C=C ● Old name: olefins • Steroids • Hormones • Biochemical regulators 2 02/21/2019 • Alkynes – Hydrocarbons containing C≡C – Common name: acetylenes Unsaturated Hydrocarbons • Arene: benzene and its derivatives (Ch 9) 3 02/21/2019 Benzene and Phenyl Groups • We do not study benzene and its derivatives until Chapter 9. – However, we show structural formulas of compounds containing a phenyl group before that time. – The phenyl group is not reactive under any of the conditions we describe in chapters 5‐8. Structure of Alkenes • The two carbon atoms of a double bond and the four atoms bonded to them lie in a plane, with bond angles of approximately 120°. 4 02/21/2019 Structure of Alkenes • Figure 4.1 According to the orbital overlap model, a double bond consists of one bond formed by overlap of sp2 hybrid orbitals and one bond formed by overlap of parallel 2p orbitals. -

Introduction to Alkenes and Alkynes in an Alkane, All Covalent Bonds
Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene, however, only three σ bonds are formed from the alkene carbon -the carbon thus adopts an sp2 hybridization Ethene (common name ethylene) has a molecular formula of CH2CH2 Each carbon is sp2 hybridized with a σ bond to two hydrogens and the other carbon Hybridized orbital allows stronger bonds due to more overlap H H C C H H Structure of Ethylene In addition to the σ framework of ethylene, each carbon has an atomic p orbital not used in hybridization The two p orbitals (each with one electron) overlap to form a π bond (p bonds are not symmetric about the internuclear axis) π bonds are not as strong as σ bonds (in ethylene, the σ bond is ~90 Kcal/mol and the π bond is ~66 Kcal/mol) Thus while σ bonds are stable and very few reactions occur with C-C bonds, π bonds are much more reactive and many reactions occur with C=C π bonds Nomenclature of Alkenes August Wilhelm Hofmann’s attempt for systematic hydrocarbon nomenclature (1866) Attempted to use a systematic name by naming all possible structures with 4 carbons Quartane a alkane C4H10 Quartyl C4H9 Quartene e alkene C4H8 Quartenyl C4H7 Quartine i alkine → alkyne C4H6 Quartinyl C4H5 Quartone o C4H4 Quartonyl C4H3 Quartune u C4H2 Quartunyl C4H1 Wanted to use Quart from the Latin for 4 – this method was not embraced and BUT has remained Used English order of vowels, however, to name the groups -

Ganic Compounds
6-1 SECTION 6 NOMENCLATURE AND STRUCTURE OF ORGANIC COMPOUNDS Many organic compounds have common names which have arisen historically, or have been given to them when the compound has been isolated from a natural product or first synthesised. As there are so many organic compounds chemists have developed rules for naming a compound systematically, so that it structure can be deduced from its name. This section introduces this systematic nomenclature, and the ways the structure of organic compounds can be depicted more simply than by full Lewis structures. The language is based on Latin, Greek and German in addition to English, so a classical education is beneficial for chemists! Greek and Latin prefixes play an important role in nomenclature: Greek Latin ½ hemi semi 1 mono uni 1½ sesqui 2 di bi 3 tri ter 4 tetra quadri 5 penta quinque 6 hexa sexi 7 hepta septi 8 octa octo 9 ennea nona 10 deca deci Organic compounds: Compounds containing the element carbon [e.g. methane, butanol]. (CO, CO2 and carbonates are classified as inorganic.) See page 1-4. Special characteristics of many organic compounds are chains or rings of carbon atoms bonded together, which provides the basis for naming, and the presence of many carbon- hydrogen bonds. The valency of carbon in organic compounds is 4. Hydrocarbons: Compounds containing only the elements C and H. Straight chain hydrocarbons are named according to the number of carbon atoms: CH4, methane; C2H6 or H3C-CH3, ethane; C3H8 or H3C-CH2-CH3, propane; C4H10 or H3C-CH2- CH2-CH3, butane; C5H12 or CH3CH2CH2CH2CH3, pentane; C6H14 or CH3(CH2)4CH3, hexane; C7H16, heptane; C8H18, octane; C9H20, nonane; C10H22, CH3(CH2)8CH3, decane. -

Reactions of Aromatic Compounds Just Like an Alkene, Benzene Has Clouds of Electrons Above and Below Its Sigma Bond Framework
Reactions of Aromatic Compounds Just like an alkene, benzene has clouds of electrons above and below its sigma bond framework. Although the electrons are in a stable aromatic system, they are still available for reaction with strong electrophiles. This generates a carbocation which is resonance stabilized (but not aromatic). This cation is called a sigma complex because the electrophile is joined to the benzene ring through a new sigma bond. The sigma complex (also called an arenium ion) is not aromatic since it contains an sp3 carbon (which disrupts the required loop of p orbitals). Ch17 Reactions of Aromatic Compounds (landscape).docx Page1 The loss of aromaticity required to form the sigma complex explains the highly endothermic nature of the first step. (That is why we require strong electrophiles for reaction). The sigma complex wishes to regain its aromaticity, and it may do so by either a reversal of the first step (i.e. regenerate the starting material) or by loss of the proton on the sp3 carbon (leading to a substitution product). When a reaction proceeds this way, it is electrophilic aromatic substitution. There are a wide variety of electrophiles that can be introduced into a benzene ring in this way, and so electrophilic aromatic substitution is a very important method for the synthesis of substituted aromatic compounds. Ch17 Reactions of Aromatic Compounds (landscape).docx Page2 Bromination of Benzene Bromination follows the same general mechanism for the electrophilic aromatic substitution (EAS). Bromine itself is not electrophilic enough to react with benzene. But the addition of a strong Lewis acid (electron pair acceptor), such as FeBr3, catalyses the reaction, and leads to the substitution product. -

Surface Chemistry Changes of Weathered HDPE/Wood-Flour
Polymer Degradation and Stability 86 (2004) 1–9 www.elsevier.com/locate/polydegstab Surface chemistry changes of weathered HDPE/wood-flour composites studied by XPS and FTIR spectroscopy* Nicole M. Starka,), Laurent M. Matuanab aU.S. Department of Agriculture, Forest Service, Forest Products Laboratory, One Gifford Pinchot Drive, Madison, WI 53726-2398, United States bDepartment of Forestry, Michigan State University, East Lansing, MI 48824-1222, United States Received 7 August 2003; received in revised form 3 November 2003; accepted 4 November 2003 Abstract The use of wood-derived fillers by the thermoplastic industry has been growing, fueled in part by the use of wood-fiber– thermoplastic composites by the construction industry. As a result, the durability of wood-fiber–thermoplastic composites after ultraviolet exposure has become a concern. Samples of 100% high-density polyethylene (HDPE) and HDPE filled with 50% wood- flour (WF) were weathered in a xenon arc-type accelerated weathering apparatus for 2000 h. Changes in surface chemistry were studied using spectroscopic techniques. X-ray photoelectron spectroscopy (XPS) was used to verify the occurrence of surface oxidation. Fourier transform infrared (FTIR) spectroscopy was used to monitor the development of degradation products, such as carbonyl groups and vinyl groups, and to determine changes in HDPE crystallinity. The results indicate that surface oxidation occurred immediately after exposure for both the neat HDPE and WF/HDPE composites; the surface of the WF/HDPE composites was oxidized to a greater extent than that of the neat HDPE. This suggests that the addition of WF to the HDPE matrix results in more weather-related damage. -

Novel Poly(Vinyl Alcohol)-Based Column Coating for Capillary Electrophoresis of Proteins
Biochemical Engineering Journal 53 (2010) 137–142 Contents lists available at ScienceDirect Biochemical Engineering Journal journal homepage: www.elsevier.com/locate/bej Novel poly(vinyl alcohol)-based column coating for capillary electrophoresis of proteins Liang Xu, Xiao-Yan Dong, Yan Sun ∗ Department of Biochemical Engineering and Key Laboratory of Systems Bioengineering of the Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China article info abstract Article history: A novel and simple method for the preparation of chemically bonded poly(vinyl alcohol) (PVA) coat- Received 30 June 2010 ing to silica capillary inner wall was developed, and the PVA-coated capillary columns were employed Received in revised form for capillary electrophoresis (CE). The coating procedure included pretreatment of the capillary inner 30 September 2010 wall, silanization, aldehyde group functionalization and PVA immobilization. Electroosmotic flow of the Accepted 6 October 2010 coated capillary was almost suppressed over a wide pH range (pH 3–10). High-efficiency separations of cationic proteins (including cytochrome c, lysozyme, ␣-chymotrypsinogen A) at pH 3.0–5.0 and of anionic proteins (including myoglobin and trypsin inhibitor) at pH 10.0 were achieved with the PVA-coated cap- Keywords: Protein illary. Moreover, a “dual-opposite-injection” approach was adopted for simultaneous separations of both Separation cationic and anionic proteins at neutral pH with the prepared column. In this CE mode, positively charged Bioprocess Monitoring proteins migrated from one end of the column to the detector while negatively charged proteins from Adsorption the other end to the detection window. Good run-to-run repeatability was obtained in all of the protein Capillary electrophoresis CE separations performed in this work. -

Chapter 7 - Alkenes and Alkynes I
Andrew Rosen Chapter 7 - Alkenes and Alkynes I 7.1 - Introduction - The simplest member of the alkenes has the common name of ethylene while the simplest member of the alkyne family has the common name of acetylene 7.2 - The (E)-(Z) System for Designating Alkene Diastereomers - To determine E or Z, look at the two groups attached to one carbon atom of the double bond and decide which has higher priority. Then, repeat this at the other carbon atom. This system is not used for cycloalkenes - If the two groups of higher priority are on the same side of the double bond, the alkene is designated Z. If the two groups of higher priority are on opposite sides of the double bond, the alkene is designated E - Hydrogenation is a syn/cis addition 7.3 - Relative Stabilities of Alkenes - The trans isomer is generally more stable than the cis isomer due to electron repulsions - The addition of hydrogen to an alkene, hydrogenation, is exothermic (heat of hydrogenation) - The greater number of attached alkyl groups, the greater the stability of an alkene 7.5 - Synthesis of Alkenes via Elimination Reactions, 7.6 - Dehydrohalogenation of Alkyl Halides How to Favor E2: - Reaction conditions that favor elimination by E1 should be avoided due to the highly competitive SN 1 mechanism - To favor E2, a secondary or tertiary alkyl halide should be used - If there is only a possibility for a primary alkyl halide, use a bulky base - Use a higher concentration of a strong and nonpolarizable base, like an alkoxide - EtONa=EtOH favors the more substituted double bond -

Reactions of Alkenes and Alkynes
05 Reactions of Alkenes and Alkynes Polyethylene is the most widely used plastic, making up items such as packing foam, plastic bottles, and plastic utensils (top: © Jon Larson/iStockphoto; middle: GNL Media/Digital Vision/Getty Images, Inc.; bottom: © Lakhesis/iStockphoto). Inset: A model of ethylene. KEY QUESTIONS 5.1 What Are the Characteristic Reactions of Alkenes? 5.8 How Can Alkynes Be Reduced to Alkenes and 5.2 What Is a Reaction Mechanism? Alkanes? 5.3 What Are the Mechanisms of Electrophilic Additions HOW TO to Alkenes? 5.1 How to Draw Mechanisms 5.4 What Are Carbocation Rearrangements? 5.5 What Is Hydroboration–Oxidation of an Alkene? CHEMICAL CONNECTIONS 5.6 How Can an Alkene Be Reduced to an Alkane? 5A Catalytic Cracking and the Importance of Alkenes 5.7 How Can an Acetylide Anion Be Used to Create a New Carbon–Carbon Bond? IN THIS CHAPTER, we begin our systematic study of organic reactions and their mecha- nisms. Reaction mechanisms are step-by-step descriptions of how reactions proceed and are one of the most important unifying concepts in organic chemistry. We use the reactions of alkenes as the vehicle to introduce this concept. 129 130 CHAPTER 5 Reactions of Alkenes and Alkynes 5.1 What Are the Characteristic Reactions of Alkenes? The most characteristic reaction of alkenes is addition to the carbon–carbon double bond in such a way that the pi bond is broken and, in its place, sigma bonds are formed to two new atoms or groups of atoms. Several examples of reactions at the carbon–carbon double bond are shown in Table 5.1, along with the descriptive name(s) associated with each. -

Gas Phase Synthesis of Interstellar Cumulenes. Mass Spectrometric and Theoretical Studies."
rl ì 6. .B,1l thesis titled: "Gas Phase Synthesis of Interstellar Cumulenes. Mass Spectrometric and Theoretical studies." submitted for the Degree of Doctor of Philosophy (Ph.D') by Stephen J. Blanksby B.Sc.(Hons,) from the Department of ChemistrY The University of Adelaide Cì UCE April 1999 Preface Contents Title page (i) Contents (ii) Abstract (v) Statement of OriginalitY (vi) Acknowledgments (vii) List of Figures (ix) Phase" I Chapter 1. "The Generation and Characterisation of Ions in the Gas 1.I Abstract I 1.II Generating ions 2 t0 l.ru The Mass SPectrometer t2 1.IV Characterisation of Ions 1.V Fragmentation Behaviour 22 Chapter 2 "Theoretical Methods for the Determination of Structure and 26 Energetics" 26 2,7 Abstract 27 2.IT Molecular Orbital Theory JJ 2.TII Density Functional Theory 2.rv Calculation of Molecular Properties 34 2.V Unimolecular Reactions 35 Chapter 3 "Interstellar and Circumstellar Cumulenes. Mass Spectrometric and 38 Related Studies" 3.I Abstract 38 3.II Interstellar Cumulenes 39 3.III Generation of Interstellar Cumulenes by Mass Spectrometry 46 3.IV Summary 59 Preface Chapter 4 "Generation of Two Isomers of C5H from the Corresponding Anions' 61 ATheoreticallyMotivatedMassSpectrometricStudy.'. 6l 4.r Abstract 62 4.rl Introduction 66 4.III Results and Discussion 83 4.IV Conclusions 84 4.V Experimental Section 89 4.VI Appendices 92 Chapter 5 "Gas Phase Syntheses of Three Isomeric CSHZ Radical Anions and Their Elusive Neutrals. A Joint Experimental and Theoretical Study." 92 5.I Abstract 93 5.II Introduction 95 5.ru Results and Discussion t12 5.IV Conclusions 113 5.V Experimental Section ttl 5.VI Appendices t20 Chapter 6 "Gas Phase Syntheses of Three Isomeric ClHz Radical Anions and Their Elusive Neutrals. -

Formation of Highly Ordered Self-Assembled Monolayers of Alkynes on Au(111) Substrate
Formation of Highly Ordered Self-Assembled Monolayers of Alkynes on Au(111) Substrate The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Zaba, Tomasz, Agnieszka Noworolska, Carleen Morris Bowers, Benjamin Breiten, George M. Whitesides, and Piotr Cyganik. 2014. Formation of Highly Ordered Self-Assembled Monolayers of Alkynes on Au(111) Substrate. Journal of the American Chemical Society 136, no. 34: 11918–11921. doi:10.1021/ja506647p. Published Version doi:10.1021/ja506647p Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:33490483 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Open Access Policy Articles, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#OAP Page 1 of 5 Journal of the American Chemical Society 1 2 3 4 5 6 7 Formation of Highly Ordered Self-Assembled Monolayers of 8 9 Alkynes on Au(111) Substrate 10 ‡ ‡ § § 11 Tomasz Zaba, Agnieszka Noworolska, Carleen Bowers, Benjamin Breiten, 12 § ‡ 13 George M. Whitesides and Piotr Cyganik* 14 ‡Smoluchowski Institute of Physics, Jagiellonian University, ul. Reymonta 4, 30-059 Krakow, Poland 15 § 16 Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138, USA 17 18 19 Supporting Information Placeholder 20 21 ABSTRACT: Self-assembled monolayers (SAMs), prepared SAMs are sensitive to oxidation at an undefined point in their formation; that is, oxidation occurs either during or after SAM 22 by reaction of terminal n-DON\QHV +&Ł& &+ 2)nCH 3, n = 5, 7, 9, o formation (for example, by UHDFWLRQ RI WKH $X&Ł&5 ERQG ZLWK 23 and 11) with Au(111) at 60 C were characterized using scanning tunneling microscopy (STM), infra-red reflection absorption O2).