Proton Nmr Spectroscopy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Espressivo Program Notes April 2018 the Evolution of Chamber Music For
Espressivo Program Notes April 2018 The evolution of chamber music for a mixed ensemble of winds and strings coincides with the domestication of the double bass. Previously used as an orchestral instrument or in dance music, the instrument was admitted into the salon in the late eighteenth century. The granddaddy of the genre is Beethoven’s Septet of 1800, for four strings, clarinet, horn and bassoon. Beethoven seems to have discovered that the largest string instrument, essentially doubling the cello an octave lower and with the capability to play six notes below the lowest note on the bassoon, provided overtones that enhanced the vibrations of the higher instruments. In any case, the popularity of the Septet, which sustains to this day, caused the original players to have a similar work commissioned from Franz Schubert, who added a second violin for his Octet (1824). (Espressivo performed it last season.) It is possible, if not probable, that Schubert’s good friend Franz Lachner wrote his Nonet for the same group, adding a flute, but the origins, and even the date of the composition are in dispute. An indicator might be the highlighting of the virtuosic violin part, as it would have been played by Ignaz Schuppanzigh, who had premiered both the Septet and the Octet. All three works, Septet, Octet and Nonet, have slow introductions to the first and last uptempo movements, and all include a minuet. All seem intended to please rather than to challenge. Beethoven’s Septet was commissioned by a noble patron for the delectation of his guests, as was Schubert’s Octet. -
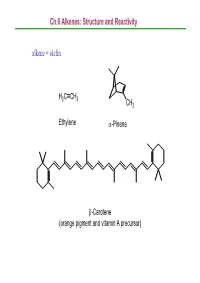
Ch.6 Alkenes: Structure and Reactivity Alkene = Olefin
Ch.6 Alkenes: Structure and Reactivity alkene = olefin H2CCH2 CH3 Ethylene α-Pinene β-Carotene (orange pigment and vitamin A precursor) Ch.6 Alkenes: Structure and Reactivity 6.1 Industrial Preparation and Use of Alkenes Compounds derived industrially from ethylene CH3CH2OH Ethanol CH3CHO Acetaldehyde CH3COOH Acetic acid HOCH2CH2OH Ethylene glycol ClCH2CH2Cl Ethylene dichloride H C=CHCl Vinyl chloride H2CCH2 2 O Ethylene oxide Ethylene (26 million tons / yr) O Vinyl acetate O Polyethylene Ch.6 Alkenes: Structure and Reactivity Compounds derived industrially from propylene OH Isopropyl alcohol H3CCH3 O Propylene oxide CH3 H3CCH CH2 Propylene Cumene (14 million tons / yr) CH3 CH3 Polypropylene Ch.6 Alkenes: Structure and Reactivity • Ethylene, propylene, and butene are synthesized industrially by thermal cracking of natural gas (C1-C4 alkanes) and straight-run gasoline (C4-C8 alkanes). 850-900oC CH (CH ) CH H + CH + H C=CH + CH CH=CH 3 2 n 3 steam 2 4 2 2 3 2 + CH3CH2CH=CH2 - the exact processes are complex; involve radical process H 900oC CH3CH2 CH2CH3 22H2CCH H2C=CH2 +H2 Ch.6 Alkenes: Structure and Reactivity • Thermal cracking is an example of a reaction whose energetics are dominated by entropy (∆So) rather than enthalpy (∆Ho) in the free-energy equation (∆Go = ∆Ho -T∆So) . ; C-C bond cleavage (positive ∆Ho) ; high T and increased number of molecules → larger T∆So Ch.6 Alkenes: Structure and Reactivity 6.2 Calculating Degree of Unsaturation unsaturated: formula of alkene CnH2n ; formula of alkane CnH2n+2 in general, each ring or double -

Spectralism in the Saxophone Repertoire: an Overview and Performance Guide
NORTHWESTERN UNIVERSITY Spectralism in the Saxophone Repertoire: An Overview and Performance Guide A PROJECT DOCUMENT SUBMITTED TO THE BIENEN SCHOOL OF MUSIC IN PARTIAL FULFILLMENT OF THE REQUIREMENTS for the degree DOCTOR OF MUSICAL ARTS Program of Saxophone Performance By Thomas Michael Snydacker EVANSTON, ILLINOIS JUNE 2019 2 ABSTRACT Spectralism in the Saxophone Repertoire: An Overview and Performance Guide Thomas Snydacker The saxophone has long been an instrument at the forefront of new music. Since its invention, supporters of the saxophone have tirelessly pushed to create a repertoire, which has resulted today in an impressive body of work for the yet relatively new instrument. The saxophone has found itself on the cutting edge of new concert music for practically its entire existence, with composers attracted both to its vast array of tonal colors and technical capabilities, as well as the surplus of performers eager to adopt new repertoire. Since the 1970s, one of the most eminent and consequential styles of contemporary music composition has been spectralism. The saxophone, predictably, has benefited tremendously, with repertoire from Gérard Grisey and other founders of the spectral movement, as well as their students and successors. Spectral music has continued to evolve and to influence many compositions into the early stages of the twenty-first century, and the saxophone, ever riding the crest of the wave of new music, has continued to expand its body of repertoire thanks in part to the influence of the spectralists. The current study is a guide for modern saxophonists and pedagogues interested in acquainting themselves with the saxophone music of the spectralists. -

Ganic Compounds
6-1 SECTION 6 NOMENCLATURE AND STRUCTURE OF ORGANIC COMPOUNDS Many organic compounds have common names which have arisen historically, or have been given to them when the compound has been isolated from a natural product or first synthesised. As there are so many organic compounds chemists have developed rules for naming a compound systematically, so that it structure can be deduced from its name. This section introduces this systematic nomenclature, and the ways the structure of organic compounds can be depicted more simply than by full Lewis structures. The language is based on Latin, Greek and German in addition to English, so a classical education is beneficial for chemists! Greek and Latin prefixes play an important role in nomenclature: Greek Latin ½ hemi semi 1 mono uni 1½ sesqui 2 di bi 3 tri ter 4 tetra quadri 5 penta quinque 6 hexa sexi 7 hepta septi 8 octa octo 9 ennea nona 10 deca deci Organic compounds: Compounds containing the element carbon [e.g. methane, butanol]. (CO, CO2 and carbonates are classified as inorganic.) See page 1-4. Special characteristics of many organic compounds are chains or rings of carbon atoms bonded together, which provides the basis for naming, and the presence of many carbon- hydrogen bonds. The valency of carbon in organic compounds is 4. Hydrocarbons: Compounds containing only the elements C and H. Straight chain hydrocarbons are named according to the number of carbon atoms: CH4, methane; C2H6 or H3C-CH3, ethane; C3H8 or H3C-CH2-CH3, propane; C4H10 or H3C-CH2- CH2-CH3, butane; C5H12 or CH3CH2CH2CH2CH3, pentane; C6H14 or CH3(CH2)4CH3, hexane; C7H16, heptane; C8H18, octane; C9H20, nonane; C10H22, CH3(CH2)8CH3, decane. -

Surface Chemistry Changes of Weathered HDPE/Wood-Flour
Polymer Degradation and Stability 86 (2004) 1–9 www.elsevier.com/locate/polydegstab Surface chemistry changes of weathered HDPE/wood-flour composites studied by XPS and FTIR spectroscopy* Nicole M. Starka,), Laurent M. Matuanab aU.S. Department of Agriculture, Forest Service, Forest Products Laboratory, One Gifford Pinchot Drive, Madison, WI 53726-2398, United States bDepartment of Forestry, Michigan State University, East Lansing, MI 48824-1222, United States Received 7 August 2003; received in revised form 3 November 2003; accepted 4 November 2003 Abstract The use of wood-derived fillers by the thermoplastic industry has been growing, fueled in part by the use of wood-fiber– thermoplastic composites by the construction industry. As a result, the durability of wood-fiber–thermoplastic composites after ultraviolet exposure has become a concern. Samples of 100% high-density polyethylene (HDPE) and HDPE filled with 50% wood- flour (WF) were weathered in a xenon arc-type accelerated weathering apparatus for 2000 h. Changes in surface chemistry were studied using spectroscopic techniques. X-ray photoelectron spectroscopy (XPS) was used to verify the occurrence of surface oxidation. Fourier transform infrared (FTIR) spectroscopy was used to monitor the development of degradation products, such as carbonyl groups and vinyl groups, and to determine changes in HDPE crystallinity. The results indicate that surface oxidation occurred immediately after exposure for both the neat HDPE and WF/HDPE composites; the surface of the WF/HDPE composites was oxidized to a greater extent than that of the neat HDPE. This suggests that the addition of WF to the HDPE matrix results in more weather-related damage. -

Novel Poly(Vinyl Alcohol)-Based Column Coating for Capillary Electrophoresis of Proteins
Biochemical Engineering Journal 53 (2010) 137–142 Contents lists available at ScienceDirect Biochemical Engineering Journal journal homepage: www.elsevier.com/locate/bej Novel poly(vinyl alcohol)-based column coating for capillary electrophoresis of proteins Liang Xu, Xiao-Yan Dong, Yan Sun ∗ Department of Biochemical Engineering and Key Laboratory of Systems Bioengineering of the Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China article info abstract Article history: A novel and simple method for the preparation of chemically bonded poly(vinyl alcohol) (PVA) coat- Received 30 June 2010 ing to silica capillary inner wall was developed, and the PVA-coated capillary columns were employed Received in revised form for capillary electrophoresis (CE). The coating procedure included pretreatment of the capillary inner 30 September 2010 wall, silanization, aldehyde group functionalization and PVA immobilization. Electroosmotic flow of the Accepted 6 October 2010 coated capillary was almost suppressed over a wide pH range (pH 3–10). High-efficiency separations of cationic proteins (including cytochrome c, lysozyme, ␣-chymotrypsinogen A) at pH 3.0–5.0 and of anionic proteins (including myoglobin and trypsin inhibitor) at pH 10.0 were achieved with the PVA-coated cap- Keywords: Protein illary. Moreover, a “dual-opposite-injection” approach was adopted for simultaneous separations of both Separation cationic and anionic proteins at neutral pH with the prepared column. In this CE mode, positively charged Bioprocess Monitoring proteins migrated from one end of the column to the detector while negatively charged proteins from Adsorption the other end to the detection window. Good run-to-run repeatability was obtained in all of the protein Capillary electrophoresis CE separations performed in this work. -

Understanding Music Past and Present
Understanding Music Past and Present N. Alan Clark, PhD Thomas Heflin, DMA Jeffrey Kluball, EdD Elizabeth Kramer, PhD Understanding Music Past and Present N. Alan Clark, PhD Thomas Heflin, DMA Jeffrey Kluball, EdD Elizabeth Kramer, PhD Dahlonega, GA Understanding Music: Past and Present is licensed under a Creative Commons Attribu- tion-ShareAlike 4.0 International License. This license allows you to remix, tweak, and build upon this work, even commercially, as long as you credit this original source for the creation and license the new creation under identical terms. If you reuse this content elsewhere, in order to comply with the attribution requirements of the license please attribute the original source to the University System of Georgia. NOTE: The above copyright license which University System of Georgia uses for their original content does not extend to or include content which was accessed and incorpo- rated, and which is licensed under various other CC Licenses, such as ND licenses. Nor does it extend to or include any Special Permissions which were granted to us by the rightsholders for our use of their content. Image Disclaimer: All images and figures in this book are believed to be (after a rea- sonable investigation) either public domain or carry a compatible Creative Commons license. If you are the copyright owner of images in this book and you have not authorized the use of your work under these terms, please contact the University of North Georgia Press at [email protected] to have the content removed. ISBN: 978-1-940771-33-5 Produced by: University System of Georgia Published by: University of North Georgia Press Dahlonega, Georgia Cover Design and Layout Design: Corey Parson For more information, please visit http://ung.edu/university-press Or email [email protected] TABLE OF C ONTENTS MUSIC FUNDAMENTALS 1 N. -

Alkynes: Molecular and Structural Formulas
Alkynes: Molecular and Structural Formulas The alkynes comprise a series of carbon- and hydrogen-based compounds that contain at least one triple bond. This group of compounds is a homologous series with the general molecular formula of C n H2 n--2 , where n equals any integer greater than one. The simplest alkyne, ethyne (also known as acetylene), has two carbon atoms and the molecular formula of C2H2. The structural formula for ethyne is In longer alkyne chains, the additional carbon atoms are attached to each other by single covalent bonds. Each carbon atom is also attached to sufficient hydrogen atoms to produce a total of four single covalent bonds about itself. In alkynes of four or more carbon atoms, the triple bond can be located in different positions along the chain, leading to the formation of structural isomers. For example, the alkyne of molecular formula C4H6 has two isomers, Although alkynes possess restricted rotation due to the triple bond, they do not have stereoisomers like the alkenes because the bonding in a carbon-carbon triple bond is sp hybridized. In sp hybridization, the maximum separation between the hybridized orbitals is 180°, so the molecule is linear. Thus, the substituents on triple-bonded carbons are positioned in a straight line, and stereoisomers are impossible Alkynes: Nomenclature Although some common alkyne names, such as acetylene, are still found in many textbooks, the International Union of Pure and Applied Chemistry (IUPAC) nomenclature is required for journal articles. The rules for alkynes in this system are identical with those for alkenes, except for the ending. -

Americanensemble
6971.american ensemble 6/14/07 2:02 PM Page 12 AmericanEnsemble Peter Serkin and the Orion String Quartet, Tishman Auditorium, April 2007 Forever Trivia question: Where Julius Levine, Isidore Cohen, Walter Trampler and David Oppenheim performed did the 12-year-old with an array of then-youngsters, including Richard Goode, Richard Stoltzman, Young Peter Serkin make his Ruth Laredo, Lee Luvisi, Murray Perahia, Jaime Laredo and Paula Robison. New York debut? The long-term viability of the New School’s low-budget, high-star-power series (Hint: The Guarneri, is due to several factors: an endowment seeded by music-loving philanthropists Cleveland, Lenox and such as Alice and Jacob Kaplan; the willingness of the participants to accept modest Vermeer string quartets made their first fees; and, of course, the New School’s ongoing generosity in providing a venue, New York appearances in the same venue.) gratis. In addition, Salomon reports, “Sasha never accepted a dime” during his 36 No, not Carnegie Recital Hall. Not the years of labor as music director or as a performer (he played in most of the 92nd Street Y, and certainly not Alice Tully concerts until 1991, two years before his death). In fact, Sasha never stopped Hall (which isn’t old enough). New Yorkers giving—the bulk of his estate went to the Schneider Foundation, which continues first heard the above-named artists in to help support the New School’s chamber music series and Schneider’s other youth- Tishman Auditorium on West 12th Street, at oriented project, the New York String Orchestra Seminar. -

Guide to Repertoire
Guide to Repertoire The chamber music repertoire is both wonderful and almost endless. Some have better grips on it than others, but all who are responsible for what the public hears need to know the landscape of the art form in an overall way, with at least a basic awareness of its details. At the end of the day, it is the music itself that is the substance of the work of both the performer and presenter. Knowing the basics of the repertoire will empower anyone who presents concerts. Here is a run-down of the meat-and-potatoes of the chamber literature, organized by instrumentation, with some historical context. Chamber music ensembles can be most simple divided into five groups: those with piano, those with strings, wind ensembles, mixed ensembles (winds plus strings and sometimes piano), and piano ensembles. Note: The listings below barely scratch the surface of repertoire available for all types of ensembles. The Major Ensembles with Piano The Duo Sonata (piano with one violin, viola, cello or wind instrument) Duo repertoire is generally categorized as either a true duo sonata (solo instrument and piano are equal partners) or as a soloist and accompanist ensemble. For our purposes here we are only discussing the former. Duo sonatas have existed since the Baroque era, and Johann Sebastian Bach has many examples, all with “continuo” accompaniment that comprises full partnership. His violin sonatas, especially, are treasures, and can be performed equally effectively with harpsichord, fortepiano or modern piano. Haydn continued to develop the genre; Mozart wrote an enormous number of violin sonatas (mostly for himself to play as he was a professional-level violinist as well). -

Memorandum LIBRARY of CONGRESS
UNITED STATES GOVERNMENT Memorandum LIBRARY OF CONGRESS 5JSC/LC/12 TO: Joint Steering Committee for Development of RDA DATE: February 6, 2008 FROM: Barbara B. Tillett, LC Representative SUBJECT: Proposed revision of RDA chap. 6, Additional instructions for musical works and expressions The Library of Congress is submitting rule revision proposals for the RDA December 2007 draft chapter 6 instructions for musical works and expressions. Goals of proposals 1. To maintain the additional instructions for music intact (although LC recommends integrating them with the general instructions after the first release of RDA). 2. To fill in gaps in the AACR2 rules carried over into RDA by a. Incorporating selected AACR2 rule revisions; b. Adding instructions that clarify, make explicit, or expand some principles and instructions carried over from AACR2; c. Proposing new instructions. 3. To simplify some unnecessarily complex instructions. 4. To arrange certain subsections in the six major instructions in a more logical way, based wherever possible on principles that group together types of resources having common characteristics. 5. To revise the instructions for medium of performance to provide for all media found in resources and to do that using vocabulary that adheres as closely as possible to the principle of representation: incorporating words the composer or resource uses. 6. To revise or eliminate instructions LC finds unworkable based on past experience with AACR2 (e.g., the instructions for key (6.22) in section M of this document). 7. In the interest of simplification, to eliminate vexing terms catalogers have spent inordinate amounts of time interpreting when using AACR2 (e.g., “type of composition” as a formal term when all it need be is a useful phrase; “score order”). -

The Dutch Connection Text Booklet
The Dutch Connection: from Utrechtsch Sextet to Hexagon Ensemble The sextet consisting of five wind instruments and piano is a colourful combination that has inspired many composers. In the 1920s there were even two professional wind sextets in the Netherlands: the Utrechtsch Sextet and the Concertgebouw Sextet. In the Concertgebouw one can still see a large painting in memory of the latter ensemble, portraying the five solo wind players from the Concertgebouw Orchestra and pianist Evert Cornelis who were members of the ensemble around 1920. Besides these sextets, there was an even larger number of wind quintets in the Netherlands. Often these ensembles were made up of first-desk wind players from symphony orchestras. For many years there were ensembles consisting of members from the Residentie Orchestra of The Hague and the Rotterdam Philharmonic Orchestra. The best known Dutch wind quintet is the Danzi Quintet, an ensemble that from the 1950s held a prominent place in Dutch music life for a long time. The ensemble not only performed music from around 1800, but also that of contemporary composers such as Jan van Vlijmen, Ton de Leeuw and Peter Schat. Some of these wind quintets were regularly augmented with a pianist in order to play sextets. The Dutch Connection is a tribute by the Hexagon Ensemble to the Dutch composers who have written sextets, as well as to the ensemble’s predecessors who performed the works. Willem Pijper (1894-1947) wrote his Sextet in the winter of 1922-1923 for the Utrechtsch Sextet, of which he was the pianist. The five wind players were first-desk players of the Utrechtsch City Orchestra.