Organic Structures 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ep 2611776 B1
(19) TZZ ___T (11) EP 2 611 776 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07D 211/36 (2006.01) C07D 471/04 (2006.01) 21.09.2016 Bulletin 2016/38 A61K 31/45 (2006.01) A61P 3/10 (2006.01) C07D 211/76 (2006.01) (21) Application number: 11822081.3 (86) International application number: (22) Date of filing: 25.08.2011 PCT/KR2011/006260 (87) International publication number: WO 2012/030106 (08.03.2012 Gazette 2012/10) (54) PRODUCTION METHOD OF INTERMEDIATE COMPOUND FOR SYNTHESIZING MEDICAMENT HERSTELLUNGSVERFAHREN FÜR EINE INTERMEDIATVERBINDUNG ZUR SYNTHESE EINES MEDIKAMENTS PROCÉDÉ DE PRODUCTION D’UN COMPOSÉ INTERMÉDIAIRE POUR LA SYNTHÈSE D’UN MÉDICAMENT (84) Designated Contracting States: EP-A2- 0 279 435 WO-A1-2006/104356 AL AT BE BG CH CY CZ DE DK EE ES FI FR GB WO-A1-2006/104356 US-A- 5 556 982 GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO US-A1- 2008 039 517 PL PT RO RS SE SI SK SM TR • KIM S ET AL: "Free Radical-Mediated (30) Priority: 03.09.2010 KR 20100086619 Carboxylation by Radical Reaction of Alkyl Iodides with Methyl Oxalyl Chloride", (43) Date of publication of application: TETRAHEDRONLETTERS, PERGAMON, GB, vol. 10.07.2013 Bulletin 2013/28 39, no. 40, 1 October 1998 (1998-10-01), pages 7317-7320, XP004133669, ISSN: 0040-4039, DOI: (73) Proprietor: LG Life Sciences Ltd 10.1016/S0040-4039(98)01568-8 Jongno-gu, Seoul 110-062 (KR) • CHRISTOPHE MORIN ET AL: "Synthesis and Evaluation of Boronated Lysine and (72) Inventors: Bis(carboranylated)[gamma]-Amino Acids as • KIM, Bong Chan Monomers for Peptide Assembly", EUROPEAN Daejeon 305-380 (KR) JOURNAL OF ORGANIC CHEMISTRY, vol. -

Acetal (POM) Chemical Compatibility Chart From
ver 31-Mar-2020 Acetal (POM) Chemical Compatibility Chart Chemical Chemical Acetaldehyde A Ammonium Acetate C Acetamide A Ammonium Bifluoride D Acetate Solvents A Ammonium Carbonate D Acetic Acid D Ammonium Caseinate D Acetic Acid, 20% C Ammonium Chloride, 10% B Acetic Acid, 80% D Ammonium Hydroxide D Acetic Acid, Glacial D Ammonium Nitrate, 10% A Acetic Anhydride D Ammonium Oxalate B Acetone A Ammonium Persulfate D Acetyl Chloride, dry D Ammonium Phosphate, Dibasic B Acetylene A Ammonium Phosphate, Monobasic B Alcohols: Amyl A Ammonium Phosphate, Tribasic B Alcohols: Benzyl A Ammonium Sulfate B Alcohols: Butyl A Ammonium Sulfite D Alcohols: Diacetone A Ammonium Thiosulfate B Alcohols: Ethyl A Amyl Acetate B Alcohols: Hexyl A Amyl Alcohol A Alcohols: Isobutyl A Amyl Chloride A Alcohols: Isopropyl A Aniline A Alcohols: Methyl A Aniline Oil D Alcohols: Octyl A Anise Oil D Alcohols: Propyl (1-Propanol) A Antifreeze D Aluminum chloride, 20% C Aqua Regia (80% HCl, 20% HNO3) D Aluminum Fluoride C Aromatic Hydrocarbons A Aluminum Hydroxide A Arsenic Acid D Aluminum Nitrate B Asphalt B Aluminum Potassium Sulfate, 10% C Barium Carbonate A Aluminum Potassium Sulfate, 100% C Barium Chloride A Aluminum Sulfate, 10% B Barium Cyanide B Alums C Barium Hydroxide D Amines D Barium Nitrate B Ammonia, 10% (Ammonium Hydroxide) C Barium Sulfate B Ammonia, 10% D Barium Sulfide A Ammonia, anhydrous D Bay Oil D Ammonia, liquid D Beer A Ammonia Nitrate C Beet Sugar Liquids B Key to General Chemical Resistance – All data is based on ambient or room temperature conditions, about 64°F (18°C) to 73°F (23°C) A = Excellent C = Fair - Moderate Effect, not recommended B= Good - Minor Effect, slight corrosion or discoloration D = Severe Effect, not recommended for ANY use It is the sole responsibility of the system designer and user to select products suitable for their specific application requirements and to ensure proper installation, operation, and maintenance of these products. -

Chapter 13 Reactions of Arenes Electrophilic Aromatic Substitution
CH. 13 Chapter 13 Reactions of Arenes Electrophilic Aromatic Substitution Electrophiles add to aromatic rings in a fashion somewhat similar to the addition of electrophiles to alkenes. Recall: R3 R4 E Y E Y C C + E Y R1 C C R4 R1 C C R4 − R2 R1 δ+ δ R2 R3 R2 R3 In aromatic rings, however, we see substitution of one of the benzene ring hydrogens for an electrophile. H E + E Y + Y H δ+ δ− The mechanism is the same regardless of the electrophile. It involves two steps: (1) Addition of the electrophile to form a high-energy carbocation. (2) Elimination of the proton to restore the aromatic ring system. H H H H slow E Y + Y step 1 + E δ+ δ− high energy arenium ion H H step 2 fast E E + Y + Y H The first step is the slow step since the aromaticity of the benzene ring system is destroyed on formation of the arenium ion intermediate. This is a high energy species but it is stabilized by resonance with the remaining two double bonds. The second step is very fast since it restores the aromatic stabilization. 1 CH. 13 H H H H H H E E E There are five electrophilic aromatic substitution reactions that we will study. (1) Nitration H NO2 H2SO4 + HNO3 (2) Sulfonation H SO3H + H2SO4 (3) Halogenation with bromine or chlorine H X FeX3 X = Br, Cl + X2 (4) Friedel-Crafts Alkylation H R AlX + RX 3 (5) Friedel-Crafts Acylation O H O C AlX3 R + Cl C R 2 CH. -
![INDOLES from 2-METHYLNITROBENZENES by CONDENSATION with FORMAMIDE ACETALS FOLLOWED by REDUCTION: 4-BENZYLOXYINDOLE [1H-Indole, 4-(Phenylmethoxy)-]](https://docslib.b-cdn.net/cover/2671/indoles-from-2-methylnitrobenzenes-by-condensation-with-formamide-acetals-followed-by-reduction-4-benzyloxyindole-1h-indole-4-phenylmethoxy-222671.webp)
INDOLES from 2-METHYLNITROBENZENES by CONDENSATION with FORMAMIDE ACETALS FOLLOWED by REDUCTION: 4-BENZYLOXYINDOLE [1H-Indole, 4-(Phenylmethoxy)-]
DOI:10.15227/orgsyn.063.0214 Organic Syntheses, Coll. Vol. 7, p.34 (1990); Vol. 63, p.214 (1985). INDOLES FROM 2-METHYLNITROBENZENES BY CONDENSATION WITH FORMAMIDE ACETALS FOLLOWED BY REDUCTION: 4-BENZYLOXYINDOLE [1H-Indole, 4-(phenylmethoxy)-] Submitted by Andrew D. Batcho1 and Willy Leimgruber2. Checked by David J. Wustrow and Andrew S. Kende. 1. Procedure A. 6-Benzyloxy-2-nitrotoluene. A stirred mixture of 124.7 g (0.81 mol) of 2-methyl-3-nitrophenol (Note 1), 113.2 g (0.90 mol) of benzyl chloride, 112.2 g (0.81 mol) of anhydrous potassium carbonate, and 800 mL of dimethylformamide (DMF) is heated at 90°C for 3 hr. Most of the DMF is removed on a rotary evaporator (20 mm) and the oily residue is poured into 400 mL of 1 N sodium hydroxide and extracted with ether (3 × 800 mL). The combined extracts are dried (Na2SO4), filtered, and evaporated to give 203.5 g of yellowish solid. Recrystallization from 1 L of methanol cooled to 0°C affords 177.6 (90%) of 6-benzyloxy-2-nitrotoluene as pale-yellow crystals, mp 61–63°C3 (Note 2). B. (E)-6-Benzyloxy-2-nitro-β-pyrrolidinostyrene. To a solution of 175.4 g (0.72 mol) of 6- benzyloxy-2-nitrotoluene in 400 mL of DMF are added 102.5 g (0.84 mol) of N,N-dimethylformamide dimethyl acetal (Note 3) and 59.8 g (0.84 mol) of pyrrolidine. The solution is heated at reflux (110°C) for 3 hr (Note 4) under nitrogen and allowed to cool to room temperature. -

Proton Nmr Spectroscopy
1H NMR Spectroscopy (#1c) The technique of 1H NMR spectroscopy is central to organic chemistry and other fields involving analysis of organic chemicals, such as forensics and environmental science. It is based on the same principle as magnetic resonance imaging (MRI). This laboratory exercise reviews the principles of interpreting 1H NMR spectra that you should be learning right now in Chemistry 302. There are four questions you should ask when you are trying to interpret an NMR spectrum. Each of these will be discussed in detail. The Four Questions to Ask While Interpreting Spectra 1. How many different environments are there? The number of peaks or resonances (signals) in the spectrum indicates the number of nonequivalent protons in a molecule. Chemically equivalent protons (magnetically equivalent protons) give the same signal in the NMR whereas nonequivalent protons give different signals. 1 For example, the compounds CH3CH3 and BrCH2CH2Br all have one peak in their H NMR spectra because all of the protons in each molecule are equivalent. The compound below, 1,2- dibromo-2-methylpropane, has two peaks: one at 1.87 ppm (the equivalent CH3’s) and the other at 3.86 ppm (the CH2). 1.87 1.87 ppm CH 3 3.86 ppm Br Br CH3 1.87 ppm 3.86 10 9 8 7 6 5 4 3 2 1 0 2. How many 1H are in each environment? The relative intensities of the signals indicate the numbers of protons that are responsible for individual signals. The area under each peak is measured in the form of an integral line. -

Handout: Organic Chemistry Reactions
Handout: Organic Chemistry Reactions Reactions Organized by Compound Families Alkanes 1. Combustion 2. Halogenation Alkenes and Alkynes 1. Additions: hydrogenation, halogenation, hydrohalogenation, hydration 2. Polymerization Aromatic Compounds Substitutions: nitration, halogenation, sulfonation Alcohols 1. Elimination: dehydration 2. Oxidations Thiols Oxidation Amines Acid-Base reactions Aldehydes and Ketones 1. Addition: Acetal/hemiacetal formation by alcohol addition (Reverse rxn: Acetal hydrolysis with acid) 2. Oxidation and Reduction for aldehydes (*Ketones go through reduction only) Carboxylic Acids 1. Substitutions: esterification, amidation (Reverse rxns: ester hydrolysis with acid or base, amide hydrolysis with acid or base) 2. Acid-base reactions Phosphoric acid and Phosphates Phosphoric acid: Esterification with alcohol Phosphates: Phosphorylation H. Kim for Chem 30B 1 Reactions Organized by Reaction Type Family Reaction Example 1. ADDITION Alkenes and Hydrogenation H H Pd catalyst H H C C + H2 H C C H alkynes H H H H H H Pd catalyst H C C H + 2 H2 H C C H H H Halogenation H H Cl Cl C C + Cl2 (or Br2) H C C H H H H H H Cl Hydro- H H Markonikov’s Rule halogenation C C + HCl (or HBr) H C C H applies: H adds to C H CH3 H CH3 with more Hs already. H OH Hydration H H H+ catalyst C C + H2O H C C H Markonikov’s Rule H CH3 H CH3 applies. Aldehydes Addition of O OH H+ catalyst H3C C H(R) + CH3CH2OH and ketones alcohol to get H3C C H(R) + CH3CH2OH hemiacetals or O CH2CH3 acetals hemiacetal O CH2CH3 H+ catalyst H3C C H(R) + H2O O CH2CH3 acetal 2. -

Fatty Acid Biosynthesis
BI/CH 422/622 ANABOLISM OUTLINE: Photosynthesis Carbon Assimilation – Calvin Cycle Carbohydrate Biosynthesis in Animals Gluconeogenesis Glycogen Synthesis Pentose-Phosphate Pathway Regulation of Carbohydrate Metabolism Anaplerotic reactions Biosynthesis of Fatty Acids and Lipids Fatty Acids contrasts Diversification of fatty acids location & transport Eicosanoids Synthesis Prostaglandins and Thromboxane acetyl-CoA carboxylase Triacylglycerides fatty acid synthase ACP priming Membrane lipids 4 steps Glycerophospholipids Control of fatty acid metabolism Sphingolipids Isoprene lipids: Cholesterol ANABOLISM II: Biosynthesis of Fatty Acids & Lipids 1 ANABOLISM II: Biosynthesis of Fatty Acids & Lipids 1. Biosynthesis of fatty acids 2. Regulation of fatty acid degradation and synthesis 3. Assembly of fatty acids into triacylglycerol and phospholipids 4. Metabolism of isoprenes a. Ketone bodies and Isoprene biosynthesis b. Isoprene polymerization i. Cholesterol ii. Steroids & other molecules iii. Regulation iv. Role of cholesterol in human disease ANABOLISM II: Biosynthesis of Fatty Acids & Lipids Lipid Fat Biosynthesis Catabolism Fatty Acid Fatty Acid Degradation Synthesis Ketone body Isoprene Utilization Biosynthesis 2 Catabolism Fatty Acid Biosynthesis Anabolism • Contrast with Sugars – Lipids have have hydro-carbons not carbo-hydrates – more reduced=more energy – Long-term storage vs short-term storage – Lipids are essential for structure in ALL organisms: membrane phospholipids • Catabolism of fatty acids –produces acetyl-CoA –produces reducing -
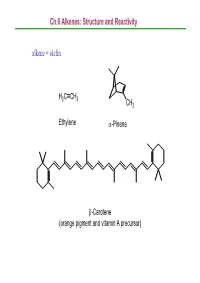
Ch.6 Alkenes: Structure and Reactivity Alkene = Olefin
Ch.6 Alkenes: Structure and Reactivity alkene = olefin H2CCH2 CH3 Ethylene α-Pinene β-Carotene (orange pigment and vitamin A precursor) Ch.6 Alkenes: Structure and Reactivity 6.1 Industrial Preparation and Use of Alkenes Compounds derived industrially from ethylene CH3CH2OH Ethanol CH3CHO Acetaldehyde CH3COOH Acetic acid HOCH2CH2OH Ethylene glycol ClCH2CH2Cl Ethylene dichloride H C=CHCl Vinyl chloride H2CCH2 2 O Ethylene oxide Ethylene (26 million tons / yr) O Vinyl acetate O Polyethylene Ch.6 Alkenes: Structure and Reactivity Compounds derived industrially from propylene OH Isopropyl alcohol H3CCH3 O Propylene oxide CH3 H3CCH CH2 Propylene Cumene (14 million tons / yr) CH3 CH3 Polypropylene Ch.6 Alkenes: Structure and Reactivity • Ethylene, propylene, and butene are synthesized industrially by thermal cracking of natural gas (C1-C4 alkanes) and straight-run gasoline (C4-C8 alkanes). 850-900oC CH (CH ) CH H + CH + H C=CH + CH CH=CH 3 2 n 3 steam 2 4 2 2 3 2 + CH3CH2CH=CH2 - the exact processes are complex; involve radical process H 900oC CH3CH2 CH2CH3 22H2CCH H2C=CH2 +H2 Ch.6 Alkenes: Structure and Reactivity • Thermal cracking is an example of a reaction whose energetics are dominated by entropy (∆So) rather than enthalpy (∆Ho) in the free-energy equation (∆Go = ∆Ho -T∆So) . ; C-C bond cleavage (positive ∆Ho) ; high T and increased number of molecules → larger T∆So Ch.6 Alkenes: Structure and Reactivity 6.2 Calculating Degree of Unsaturation unsaturated: formula of alkene CnH2n ; formula of alkane CnH2n+2 in general, each ring or double -

Carboxylic Acid Functionalized Butyl Rubber: from Synthesis to Applications
Western University Scholarship@Western Electronic Thesis and Dissertation Repository 2-7-2013 12:00 AM Carboxylic Acid Functionalized Butyl Rubber: From Synthesis to Applications Matthew John McEachran The University of Western Ontario Supervisor Dr. Elizabeth R. Gillies The University of Western Ontario Graduate Program in Chemistry A thesis submitted in partial fulfillment of the equirr ements for the degree in Master of Science © Matthew John McEachran 2013 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Materials Chemistry Commons, Medicinal-Pharmaceutical Chemistry Commons, and the Polymer Chemistry Commons Recommended Citation McEachran, Matthew John, "Carboxylic Acid Functionalized Butyl Rubber: From Synthesis to Applications" (2013). Electronic Thesis and Dissertation Repository. 1117. https://ir.lib.uwo.ca/etd/1117 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. Carboxylic Acid Functionalized Butyl Rubber: From Synthesis to Applications (Spine title: Carboxylic Acid Functionalized Butyl Rubber) (Thesis format: Integrated Article) by Matthew John McEachran Graduate Program in Chemistry A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science The School of Graduate and Postdoctoral Studies The University of Western Ontario London, -

Benzyl Ligand† Cite This: Chem
ChemComm View Article Online COMMUNICATION View Journal | View Issue Homoleptic organolanthanide compounds supported by the bis(dimethylsilyl)benzyl ligand† Cite this: Chem. Commun., 2017, 53,716 Kasuni C. Boteju, Arkady Ellern and Aaron D. Sadow* Received 21st November 2016, Accepted 12th December 2016 DOI: 10.1039/c6cc09304c www.rsc.org/chemcomm À A b-SiH functionalized benzyl anion [C(SiHMe2)2Ph] is obtained by A strategy for stabilizing coordinatively unsaturated rare earth deprotonation of HC(SiHMe2)2Ph with KCH2Ph or by reaction of amides has involved the incorporation of SiH groups, which 14 KOtBu and (Me2HSi)3CPh; LnI3(THF)n and three equivalents of this form labile secondary interactions with the lanthanide center. carbanion combine to provide homoleptic tris(alkyl)lanthanide Furthermore, the SiH moiety provides a powerful signature in 1 29 Creative Commons Attribution 3.0 Unported Licence. compounds Ln{C(SiHMe2)2Ph}3 (Ln = La, Ce, Pr, Nd) containing Hand Si NMR and IR spectra. This b-SiH strategy may also be secondary metal–ligand interactions. applied to alkyls, and the ligand C(SiHMe2)3 supports trivalent yttrium and divalent ytterbium and samarium homoleptic alkyls Synthesis of homoleptic organolanthanide complexes, particu- containing secondary Ln(H–Si interactions.15,16 Recently, we larly those of the early trivalent lanthanides (La–Nd), is challenging reported Ce{C(SiHMe2)3}3 as a precursor to a zwitterionic hydro- due to the large radii of these elements, polar bonding, high silylation catalyst.17 New chemistry might be accessed with alkyl charge, and high Lewis acidity.1 Such homoleptic compounds ligand variations that include both b-SiH and benzylic function- should be valuable for the synthesis of new catalysts and alities, and these groups could compete to enhance the homo- new materials,2 yet solvent- or donor-group-free, salt-free, and leptic compounds’ resistance to undesired ligand elimination This article is licensed under a thermally robust organolanthanide compounds are not readily pathways. -

Ganic Compounds
6-1 SECTION 6 NOMENCLATURE AND STRUCTURE OF ORGANIC COMPOUNDS Many organic compounds have common names which have arisen historically, or have been given to them when the compound has been isolated from a natural product or first synthesised. As there are so many organic compounds chemists have developed rules for naming a compound systematically, so that it structure can be deduced from its name. This section introduces this systematic nomenclature, and the ways the structure of organic compounds can be depicted more simply than by full Lewis structures. The language is based on Latin, Greek and German in addition to English, so a classical education is beneficial for chemists! Greek and Latin prefixes play an important role in nomenclature: Greek Latin ½ hemi semi 1 mono uni 1½ sesqui 2 di bi 3 tri ter 4 tetra quadri 5 penta quinque 6 hexa sexi 7 hepta septi 8 octa octo 9 ennea nona 10 deca deci Organic compounds: Compounds containing the element carbon [e.g. methane, butanol]. (CO, CO2 and carbonates are classified as inorganic.) See page 1-4. Special characteristics of many organic compounds are chains or rings of carbon atoms bonded together, which provides the basis for naming, and the presence of many carbon- hydrogen bonds. The valency of carbon in organic compounds is 4. Hydrocarbons: Compounds containing only the elements C and H. Straight chain hydrocarbons are named according to the number of carbon atoms: CH4, methane; C2H6 or H3C-CH3, ethane; C3H8 or H3C-CH2-CH3, propane; C4H10 or H3C-CH2- CH2-CH3, butane; C5H12 or CH3CH2CH2CH2CH3, pentane; C6H14 or CH3(CH2)4CH3, hexane; C7H16, heptane; C8H18, octane; C9H20, nonane; C10H22, CH3(CH2)8CH3, decane. -

Aldehydes Can React with Alcohols to Form Hemiacetals
340 14 . Nucleophilic substitution at C=O with loss of carbonyl oxygen You have, in fact, already met some reactions in which the carbonyl oxygen atom can be lost, but you probably didn’t notice at the time. The equilibrium between an aldehyde or ketone and its hydrate (p. 000) is one such reaction. O HO OH H2O + R1 R2 R1 R2 When the hydrate reverts to starting materials, either of its two oxygen atoms must leave: one OPh came from the water and one from the carbonyl group, so 50% of the time the oxygen atom that belonged to the carbonyl group will be lost. Usually, this is of no consequence, but it can be useful. O For example, in 1968 some chemists studying the reactions that take place inside mass spectrometers needed to label the carbonyl oxygen atom of this ketone with the isotope 18 O. 16 18 By stirring the ‘normal’ O compound with a large excess of isotopically labelled water, H 2 O, for a few hours in the presence of a drop of acid they were able to make the required labelled com- í In Chapter 13 we saw this way of pound. Without the acid catalyst, the exchange is very slow. Acid catalysis speeds the reaction up by making a reaction go faster by raising making the carbonyl group more electrophilic so that equilibrium is reached more quickly. The the energy of the starting material. We 18 also saw that the position of an equilibrium is controlled by mass action— O is in large excess.