Ganic Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cinnamoyl Esters of Lesquerella and Castor Oil: Novel Sunscreen Active Ingredients David L
Cinnamoyl Esters of Lesquerella and Castor Oil: Novel Sunscreen Active Ingredients David L. Compton*, Joseph A. Laszlo, and Terry A. Isbell New Crops and Processing Technology Research Unit, USDA, ARS, NCAUR, Peoria, Illinois 61604 ABSTRACT: Lesquerella and castor oils were esterified with querolin (2). Therefore, LO in essence contains two moles of cinnamic acid (CA) and 4-methoxycinnamic acid (MCA). Esteri- hydroxy functionality per mole of triglyceride (TG). fication of the hydroxy oils reached 85% completion with CA The hydroxy functionality of the FA of the TG can be ex- and 50% conversion with MCA. The hydroxy oils were esteri- ploited by esterification to form estolides. The majority of re- fied at 200°C under a nitrogen atmosphere within a sealed sys- search has focused on the estolides of hydroxy FA and TG tem. Unreacted CA and MCA were removed from the reaction formed with oleic acid. The esterification of hydroxy TG and mixtures by sublimation at 100°C under vacuum. The resultant free lesquerolic acid with oleic acid using a cobalt catalyst (4) methoxycinnamic oils possessed a broader, more blue-shifted or a lipase catalyst (5) has been reported. Also, the acid-cat- UV absorbance, 250 to 345 nm with a λmax of 305 nm, com- pared with the cinnamic oils, which absorbed from 260 to 315 alyzed formation of estolides from CO and LO with oleic acid has been patented (6). Recently, a detailed study of the effect nm, λmax of 270 nm. The methoxycinnamic oils provide better UV-B absorption and thus are better candidates to be used as of oleic acid concentration and temperature on catalyst-free sunscreen active ingredients. -

Chapter 13 Reactions of Arenes Electrophilic Aromatic Substitution
CH. 13 Chapter 13 Reactions of Arenes Electrophilic Aromatic Substitution Electrophiles add to aromatic rings in a fashion somewhat similar to the addition of electrophiles to alkenes. Recall: R3 R4 E Y E Y C C + E Y R1 C C R4 R1 C C R4 − R2 R1 δ+ δ R2 R3 R2 R3 In aromatic rings, however, we see substitution of one of the benzene ring hydrogens for an electrophile. H E + E Y + Y H δ+ δ− The mechanism is the same regardless of the electrophile. It involves two steps: (1) Addition of the electrophile to form a high-energy carbocation. (2) Elimination of the proton to restore the aromatic ring system. H H H H slow E Y + Y step 1 + E δ+ δ− high energy arenium ion H H step 2 fast E E + Y + Y H The first step is the slow step since the aromaticity of the benzene ring system is destroyed on formation of the arenium ion intermediate. This is a high energy species but it is stabilized by resonance with the remaining two double bonds. The second step is very fast since it restores the aromatic stabilization. 1 CH. 13 H H H H H H E E E There are five electrophilic aromatic substitution reactions that we will study. (1) Nitration H NO2 H2SO4 + HNO3 (2) Sulfonation H SO3H + H2SO4 (3) Halogenation with bromine or chlorine H X FeX3 X = Br, Cl + X2 (4) Friedel-Crafts Alkylation H R AlX + RX 3 (5) Friedel-Crafts Acylation O H O C AlX3 R + Cl C R 2 CH. -

Proton Nmr Spectroscopy
1H NMR Spectroscopy (#1c) The technique of 1H NMR spectroscopy is central to organic chemistry and other fields involving analysis of organic chemicals, such as forensics and environmental science. It is based on the same principle as magnetic resonance imaging (MRI). This laboratory exercise reviews the principles of interpreting 1H NMR spectra that you should be learning right now in Chemistry 302. There are four questions you should ask when you are trying to interpret an NMR spectrum. Each of these will be discussed in detail. The Four Questions to Ask While Interpreting Spectra 1. How many different environments are there? The number of peaks or resonances (signals) in the spectrum indicates the number of nonequivalent protons in a molecule. Chemically equivalent protons (magnetically equivalent protons) give the same signal in the NMR whereas nonequivalent protons give different signals. 1 For example, the compounds CH3CH3 and BrCH2CH2Br all have one peak in their H NMR spectra because all of the protons in each molecule are equivalent. The compound below, 1,2- dibromo-2-methylpropane, has two peaks: one at 1.87 ppm (the equivalent CH3’s) and the other at 3.86 ppm (the CH2). 1.87 1.87 ppm CH 3 3.86 ppm Br Br CH3 1.87 ppm 3.86 10 9 8 7 6 5 4 3 2 1 0 2. How many 1H are in each environment? The relative intensities of the signals indicate the numbers of protons that are responsible for individual signals. The area under each peak is measured in the form of an integral line. -

Unit One Part 2: Naming and Functional Groups
gjr-–- 1 Unit One Part 2: naming and functional groups • To write and interpret IUPAC names for small, simple molecules • Identify some common functional groups found in organic molecules O CH3 H3C N H N O N N O H3C S N O N H3C viagra™ (trade name) sildenafil (trivial name) 5-(2-ethoxy-5-(4-methylpiperazin-1-ylsulfonyl)phenyl)-1-methyl-3-propyl-1H- pyrazolo[4,3-d] pyrimidin-7(6H)-one dr gareth rowlands; [email protected]; science tower a4.12 http://www.massey.ac.nz/~gjrowlan gjr-–- 2 Systematic (IUPAC) naming PREFIX PARENT SUFFIX substituents / minor number of C principal functional functional groups AND multiple bond group index • Comprises of three main parts • Note: multiple bond index is always incorporated in parent section No. Carbons Root No. Carbons Root Multiple-bond 1 meth 6 hex Bond index 2 eth 7 hept C–C an(e) 3 prop 8 oct C=C en(e) 4 but 9 non C≡C yn(e) 5 pent 10 dec gjr-–- 3 Systematic (IUPAC) naming: functional groups Functional group Structure Suffix Prefix General form O –oic acid acid –carboxylic acid carboxy R-COOH R OH O O –oic anhydride anhydride –carboxylic anhydride R-C(O)OC(O)-R R O R O –oyl chloride acyl chloride -carbonyl chloride chlorocarbonyl R-COCl R Cl O –oate ester –carboxylate alkoxycarbonyl R-COOR R OR O –amide amide –carboxamide carbamoyl R-CONH2 R NH2 nitrile R N –nitrile cyano R-C≡N O –al aldehyde –carbaldehyde oxo R-CHO R H O ketone –one oxo R-CO-R R R alcohol R OH –ol hydroxy R-OH amine R NH2 –amine amino R-NH2 O ether R R –ether alkoxy R-O-R alkyl bromide bromo R-Br (alkyl halide) R Br (halo) (R-X) gjr-–- 4 Nomenclature rules 1. -

Polymer Exemption Guidance Manual POLYMER EXEMPTION GUIDANCE MANUAL
United States Office of Pollution EPA 744-B-97-001 Environmental Protection Prevention and Toxics June 1997 Agency (7406) Polymer Exemption Guidance Manual POLYMER EXEMPTION GUIDANCE MANUAL 5/22/97 A technical manual to accompany, but not supersede the "Premanufacture Notification Exemptions; Revisions of Exemptions for Polymers; Final Rule" found at 40 CFR Part 723, (60) FR 16316-16336, published Wednesday, March 29, 1995 Environmental Protection Agency Office of Pollution Prevention and Toxics 401 M St., SW., Washington, DC 20460-0001 Copies of this document are available through the TSCA Assistance Information Service at (202) 554-1404 or by faxing requests to (202) 554-5603. TABLE OF CONTENTS LIST OF EQUATIONS............................ ii LIST OF FIGURES............................. ii LIST OF TABLES ............................. ii 1. INTRODUCTION ............................ 1 2. HISTORY............................... 2 3. DEFINITIONS............................. 3 4. ELIGIBILITY REQUIREMENTS ...................... 4 4.1. MEETING THE DEFINITION OF A POLYMER AT 40 CFR §723.250(b)... 5 4.2. SUBSTANCES EXCLUDED FROM THE EXEMPTION AT 40 CFR §723.250(d) . 7 4.2.1. EXCLUSIONS FOR CATIONIC AND POTENTIALLY CATIONIC POLYMERS ....................... 8 4.2.1.1. CATIONIC POLYMERS NOT EXCLUDED FROM EXEMPTION 8 4.2.2. EXCLUSIONS FOR ELEMENTAL CRITERIA........... 9 4.2.3. EXCLUSIONS FOR DEGRADABLE OR UNSTABLE POLYMERS .... 9 4.2.4. EXCLUSIONS BY REACTANTS................ 9 4.2.5. EXCLUSIONS FOR WATER-ABSORBING POLYMERS........ 10 4.3. CATEGORIES WHICH ARE NO LONGER EXCLUDED FROM EXEMPTION .... 10 4.4. MEETING EXEMPTION CRITERIA AT 40 CFR §723.250(e) ....... 10 4.4.1. THE (e)(1) EXEMPTION CRITERIA............. 10 4.4.1.1. LOW-CONCERN FUNCTIONAL GROUPS AND THE (e)(1) EXEMPTION................. -

Fatty Acid Biosynthesis
BI/CH 422/622 ANABOLISM OUTLINE: Photosynthesis Carbon Assimilation – Calvin Cycle Carbohydrate Biosynthesis in Animals Gluconeogenesis Glycogen Synthesis Pentose-Phosphate Pathway Regulation of Carbohydrate Metabolism Anaplerotic reactions Biosynthesis of Fatty Acids and Lipids Fatty Acids contrasts Diversification of fatty acids location & transport Eicosanoids Synthesis Prostaglandins and Thromboxane acetyl-CoA carboxylase Triacylglycerides fatty acid synthase ACP priming Membrane lipids 4 steps Glycerophospholipids Control of fatty acid metabolism Sphingolipids Isoprene lipids: Cholesterol ANABOLISM II: Biosynthesis of Fatty Acids & Lipids 1 ANABOLISM II: Biosynthesis of Fatty Acids & Lipids 1. Biosynthesis of fatty acids 2. Regulation of fatty acid degradation and synthesis 3. Assembly of fatty acids into triacylglycerol and phospholipids 4. Metabolism of isoprenes a. Ketone bodies and Isoprene biosynthesis b. Isoprene polymerization i. Cholesterol ii. Steroids & other molecules iii. Regulation iv. Role of cholesterol in human disease ANABOLISM II: Biosynthesis of Fatty Acids & Lipids Lipid Fat Biosynthesis Catabolism Fatty Acid Fatty Acid Degradation Synthesis Ketone body Isoprene Utilization Biosynthesis 2 Catabolism Fatty Acid Biosynthesis Anabolism • Contrast with Sugars – Lipids have have hydro-carbons not carbo-hydrates – more reduced=more energy – Long-term storage vs short-term storage – Lipids are essential for structure in ALL organisms: membrane phospholipids • Catabolism of fatty acids –produces acetyl-CoA –produces reducing -

Studies on the Chemistry of Paclitaxel
STUDIES ON THE CHEMISTRY OF PACLITAXEL Haiqing Yuan Dissertation submitted to the Faculty of the Virginia Polytechnic Institute and State University in the partial fulfillment of the requirement for the degree of Doctor of Philosophy in Chemistry Dr. David G. I. Kingston, Chair Dr. Michael Calter Dr. Neal Castagnoli, Jr. Dr. Richard Gandour Dr. Larry Taylor August 11, 1998 Blacksburg, Virginia Keywords: Paclitaxel, Taxol®, synthesis, analog, SAR Copyright 1998, Haiqing Yuan STUDIES ON THE CHEMISTRY OF PACLITAXEL HAIQING YUAN (ABSTRACT) Paclitaxel is a natural occurring diterpene alkaloid originally isolated from the bark of Taxus brevifolia. It is now one of the most important chemotherapeutic agents for clinical treatment of ovarian and breast cancers. Recent clinical trials have also shown paclitaxel’s potential for the treatment of non-small-cell lung cancer, head and neck cancer, and other types of cancers. While tremendous chemical research efforts have been made in the past years, which established the fundamental structure-activity relationships of the paclitaxel molecule, and provided analogs for biochemical studies to elucidate the precise mechanism of action and for the development of second-generation agents, many areas remain to be explored. In continuation of our efforts in the structure-activity relationships study of A- norpaclitaxel, five new analogs modified at the C-1 substituent and analogs with expanded B-ring or contracted C-ring have now been prepared. Preliminary biological studies indicated that the volume rather than functionality at the C-1 position plays a role in determining the anticancer activity by controlling the relative position of the tetracyclic ring system, which in turn controls the positions of the most critical functionalities such as the C-2 benzoyl, the C-4 acetate, and the C-13 side chain. -
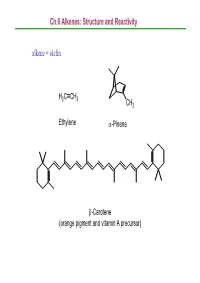
Ch.6 Alkenes: Structure and Reactivity Alkene = Olefin
Ch.6 Alkenes: Structure and Reactivity alkene = olefin H2CCH2 CH3 Ethylene α-Pinene β-Carotene (orange pigment and vitamin A precursor) Ch.6 Alkenes: Structure and Reactivity 6.1 Industrial Preparation and Use of Alkenes Compounds derived industrially from ethylene CH3CH2OH Ethanol CH3CHO Acetaldehyde CH3COOH Acetic acid HOCH2CH2OH Ethylene glycol ClCH2CH2Cl Ethylene dichloride H C=CHCl Vinyl chloride H2CCH2 2 O Ethylene oxide Ethylene (26 million tons / yr) O Vinyl acetate O Polyethylene Ch.6 Alkenes: Structure and Reactivity Compounds derived industrially from propylene OH Isopropyl alcohol H3CCH3 O Propylene oxide CH3 H3CCH CH2 Propylene Cumene (14 million tons / yr) CH3 CH3 Polypropylene Ch.6 Alkenes: Structure and Reactivity • Ethylene, propylene, and butene are synthesized industrially by thermal cracking of natural gas (C1-C4 alkanes) and straight-run gasoline (C4-C8 alkanes). 850-900oC CH (CH ) CH H + CH + H C=CH + CH CH=CH 3 2 n 3 steam 2 4 2 2 3 2 + CH3CH2CH=CH2 - the exact processes are complex; involve radical process H 900oC CH3CH2 CH2CH3 22H2CCH H2C=CH2 +H2 Ch.6 Alkenes: Structure and Reactivity • Thermal cracking is an example of a reaction whose energetics are dominated by entropy (∆So) rather than enthalpy (∆Ho) in the free-energy equation (∆Go = ∆Ho -T∆So) . ; C-C bond cleavage (positive ∆Ho) ; high T and increased number of molecules → larger T∆So Ch.6 Alkenes: Structure and Reactivity 6.2 Calculating Degree of Unsaturation unsaturated: formula of alkene CnH2n ; formula of alkane CnH2n+2 in general, each ring or double -

Organic Chemistry Tests for Hydroxyl Group
Chemistry Organic Chemistry Tests for Hydroxyl Group General Aim Method Identication of aliphatic alcohols through the chemical Detection of the presence of hydroxyl groups in detection of hydroxyl groups. aliphatic alcohols using special chemical tests. Learning Objectives (ILOs) Dene and determine aliphatic alcohols theoretically through their chemical structure. Classify organic compounds containing hydroxyl groups into aliphatic and aromatic. Compare between alcohols and other functional groups in terms of chemical structures, properties and reactions. Identify aliphatic alcohols experimentally. Select the appropriate reagents to dierentiate between alcohols and other organic compounds. Theoretical Background/Context - Aliphatic alcohols are non-aromatic hydrocarbons possessing at least one hydroxyl group within their structure. They can be either cyclic or acyclic compounds. Alcohols are considered to be neutral compounds. Aliphatic Cyclic Alcohol Aliphatic Acyclic Alcohol - Alcohols are also classied to primary, secondary and tertiary alcohols according to the number of carbon atoms attached to the carbon atom linked to the hydroxyl group. First: Preparation of Aliphatic Alcohols - Alcohols can be prepared through some chemical routes such as reduction of the corresponding aldehydes and ketones using some reducing agents such as lithium aluminum hydride or sodium borohydride. They can be also obtained from hydration of the corresponding alkenes. Some primary alcohols can be synthesized through the nucleophilic substitution of corresponding alkyl halides using potassium or sodium hydroxides. - Alcohols can be also obtained through some biological routes such as ethanol and butanol through fermentation processes in presence of glucose. Glucose is obtained from starch hydrolysis in the presence of yeast. 1 www.praxilabs.com Theoretical Background/Context (Cont’) Second: General Properties of Aliphatic Alcohols - Aliphatic alcohols are polar compounds and can form hydrogen bonds easily. -

Benzyl Ligand† Cite This: Chem
ChemComm View Article Online COMMUNICATION View Journal | View Issue Homoleptic organolanthanide compounds supported by the bis(dimethylsilyl)benzyl ligand† Cite this: Chem. Commun., 2017, 53,716 Kasuni C. Boteju, Arkady Ellern and Aaron D. Sadow* Received 21st November 2016, Accepted 12th December 2016 DOI: 10.1039/c6cc09304c www.rsc.org/chemcomm À A b-SiH functionalized benzyl anion [C(SiHMe2)2Ph] is obtained by A strategy for stabilizing coordinatively unsaturated rare earth deprotonation of HC(SiHMe2)2Ph with KCH2Ph or by reaction of amides has involved the incorporation of SiH groups, which 14 KOtBu and (Me2HSi)3CPh; LnI3(THF)n and three equivalents of this form labile secondary interactions with the lanthanide center. carbanion combine to provide homoleptic tris(alkyl)lanthanide Furthermore, the SiH moiety provides a powerful signature in 1 29 Creative Commons Attribution 3.0 Unported Licence. compounds Ln{C(SiHMe2)2Ph}3 (Ln = La, Ce, Pr, Nd) containing Hand Si NMR and IR spectra. This b-SiH strategy may also be secondary metal–ligand interactions. applied to alkyls, and the ligand C(SiHMe2)3 supports trivalent yttrium and divalent ytterbium and samarium homoleptic alkyls Synthesis of homoleptic organolanthanide complexes, particu- containing secondary Ln(H–Si interactions.15,16 Recently, we larly those of the early trivalent lanthanides (La–Nd), is challenging reported Ce{C(SiHMe2)3}3 as a precursor to a zwitterionic hydro- due to the large radii of these elements, polar bonding, high silylation catalyst.17 New chemistry might be accessed with alkyl charge, and high Lewis acidity.1 Such homoleptic compounds ligand variations that include both b-SiH and benzylic function- should be valuable for the synthesis of new catalysts and alities, and these groups could compete to enhance the homo- new materials,2 yet solvent- or donor-group-free, salt-free, and leptic compounds’ resistance to undesired ligand elimination This article is licensed under a thermally robust organolanthanide compounds are not readily pathways. -

Mann Et Al. Text 22
Catalytic Z-Selective Cross-Metathesis with Secondary Silyl- and Benzyl-Protected Allylic Ethers: Mechanistic Aspects and Applications to Natural Product Synthesis The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Mann, Tyler J., Alexander W. H. Speed, Richard R. Schrock, and Amir H. Hoveyda. “Catalytic Z-Selective Cross-Metathesis with Secondary Silyl- and Benzyl-Protected Allylic Ethers: Mechanistic Aspects and Applications to Natural Product Synthesis.” Angewandte Chemie International Edition 52, no. 32 (August 5, 2013): 8395-8400. As Published http://dx.doi.org/10.1002/anie.201302538 Publisher Wiley Blackwell Version Original manuscript Citable link http://hdl.handle.net/1721.1/84085 Terms of Use Creative Commons Attribution-Noncommercial-Share Alike 3.0 Detailed Terms http://creativecommons.org/licenses/by-nc-sa/3.0/ Catalytic Z-Selective Cross-Metathesis with Secondary Silyl- and Benzyl-Protected Allylic Ethers: Mechanistic Aspects and Applications to Natural Product Synthesis** Tyler J. Mann, Alexander W. H. Speed, Richard R. Schrock and Amir H. Hoveyda* [*] Prof. A. H. Hoveyda, T. J. Mann, Dr. A. W. H. Speed Department of Chemistry, Merkert Chemistry Center, Boston College, Chestnut Hill, MA 02467 (USA) Fax: (1) 617-552-1442 E-mail: [email protected] Prof. R. R. Schrock Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139 (USA) [**] Financial support was provided by the NIH (GM-59426). We are grateful to Robert V. O’Brien -

Surface Chemistry Changes of Weathered HDPE/Wood-Flour
Polymer Degradation and Stability 86 (2004) 1–9 www.elsevier.com/locate/polydegstab Surface chemistry changes of weathered HDPE/wood-flour composites studied by XPS and FTIR spectroscopy* Nicole M. Starka,), Laurent M. Matuanab aU.S. Department of Agriculture, Forest Service, Forest Products Laboratory, One Gifford Pinchot Drive, Madison, WI 53726-2398, United States bDepartment of Forestry, Michigan State University, East Lansing, MI 48824-1222, United States Received 7 August 2003; received in revised form 3 November 2003; accepted 4 November 2003 Abstract The use of wood-derived fillers by the thermoplastic industry has been growing, fueled in part by the use of wood-fiber– thermoplastic composites by the construction industry. As a result, the durability of wood-fiber–thermoplastic composites after ultraviolet exposure has become a concern. Samples of 100% high-density polyethylene (HDPE) and HDPE filled with 50% wood- flour (WF) were weathered in a xenon arc-type accelerated weathering apparatus for 2000 h. Changes in surface chemistry were studied using spectroscopic techniques. X-ray photoelectron spectroscopy (XPS) was used to verify the occurrence of surface oxidation. Fourier transform infrared (FTIR) spectroscopy was used to monitor the development of degradation products, such as carbonyl groups and vinyl groups, and to determine changes in HDPE crystallinity. The results indicate that surface oxidation occurred immediately after exposure for both the neat HDPE and WF/HDPE composites; the surface of the WF/HDPE composites was oxidized to a greater extent than that of the neat HDPE. This suggests that the addition of WF to the HDPE matrix results in more weather-related damage.