No. of Carbons IUPAC Name Common Name Formula Cnh2n
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Study of the Thermal Stability of Benzyl 2,4-Dimethyl-6
A STUDY OF THE THERMAL STABILITY OF BENZYL 2 1 4·DIMETHYL•6•PROPENYLPEENYL ETHER by RONALD JENE DUPZIK A THESIS submitted to OREGON STATE COLLEGE in partial fulfillment ot the requirements tor the degree ot MASTER OF SCIENCE June 1958 APPEOYED I Redacted for Privacy tarogl,rtr Frofrlter of 0rnt In 0hrrgr of, I{aJor Redacted for Privacy Chrlrnen of Drprntmat of Shontrtrf Redacted for Privacy Ghelrura of Bahool &adurtc 0omlttm Redacted for Privacy Deur of 0mdu*tr $rhoel Drtc thrrtr lr prrrontrd Ausust 7, 1957 Sypcd by l{*y ldrnr 'l'o M;y Mothez in memory ot My Father ACKNOWLEDGMENT The author wishes to expreaa hia apprecia tion to Dr. Elliot N. Marvell whose help and guidance has made this work possible. Thanks are given to Dr. B. E. Christensen and Dr . c. s. Pease who have so graciously helped in Dr. Marvell's absence. To Miss Isabelle Forbusoh for her patience and help in the preparation of this manuscript. And, to the Martinez Research Laboratory ot the Shell Oil Company for the infrared and ultraviolet spectral analysis of the new compounds. TABLE OF CONTENTS Pa!e INTRODUCTION • • • • • • • • • • • • • • • • • • 1 HISTORICAL • • • • • • • • • • • . •. • • • • • • 3 DISCUSSION • • • • • • • • • • • • • • • • • • • • 16 EXPERIMENTAL • • • • • • • • • • • • • • • • • • 29 Synthesis A • • • • • • 29 Synthesis B • • • • • • • • • • • • • • • 31 Rearrangement and Thermal Stability • • • • • 37 SUMMARY. • • • • • • • • • • • • • 40 BI BLIOGRAPHY • • • • • • • • • • • • • • • • • • • 41 LIST OF FIGURES Figure 1 • • • " • • • • • • • • • • -

Allicin in Blood.Pdf
European Journal of Medicinal Chemistry 45 (2010) 1912–1918 Contents lists available at ScienceDirect European Journal of Medicinal Chemistry journal homepage: http://www.elsevier.com/locate/ejmech Original article Reaction mechanisms of allicin and allyl-mixed disulfides with proteins and small thiol molecules Talia Miron a,*, Irving Listowsky b, Meir Wilchek a a Department of Biological Chemistry, Weizmann Institute of Science, Rehovot 76100, Israel b Department of Biochemistry, Albert-Einstein College of Medicine, Bronx, NY, USA article info abstract Article history: Allylsulfides from garlic are chemopreventive agents. Entering cells they are expected to initially interact Received 15 October 2009 with glutathione. Accordingly, reaction mechanisms of the product, S-allylthio-glutathione, with model Received in revised form proteins and thiols were analyzed in cell free systems. With glutathionyl, cysteinyl or captopril repre- 14 January 2010 senting S-allyl aliphatic adducts, the reaction with sulfhydryl groups resulted in mixed disulfide Accepted 15 January 2010 mixtures, formed by both, S-allyl and aliphatic moieties. Available online 21 January 2010 To improve conventional prodrug treatment of blood pressure, cancer and intestinal inflammation S-allylthio prodrugs, such as S-allylthio-6-mercaptopurine and S-allylthio-captopril were synthesized. Keywords: Allicin Synergistic activities of the 2 constituents, as well as increased cell permeability allow for efficient in vivo Glutathione activity. Upon reaction of these derivatives with glutathione, S-allylthio-glutathione is formed, while S-Allylthio-mixed disulfide 6-mercaptopurine is the leaving group. Excess cellular glutathione enables several cycles of sulfhydryl- Prodrug disulfide exchange reactions to occur, extending the hybrid drug’s pharmacodynamics. Mechanism of action Ó 2010 Elsevier Masson SAS. -

Proton Nmr Spectroscopy
1H NMR Spectroscopy (#1c) The technique of 1H NMR spectroscopy is central to organic chemistry and other fields involving analysis of organic chemicals, such as forensics and environmental science. It is based on the same principle as magnetic resonance imaging (MRI). This laboratory exercise reviews the principles of interpreting 1H NMR spectra that you should be learning right now in Chemistry 302. There are four questions you should ask when you are trying to interpret an NMR spectrum. Each of these will be discussed in detail. The Four Questions to Ask While Interpreting Spectra 1. How many different environments are there? The number of peaks or resonances (signals) in the spectrum indicates the number of nonequivalent protons in a molecule. Chemically equivalent protons (magnetically equivalent protons) give the same signal in the NMR whereas nonequivalent protons give different signals. 1 For example, the compounds CH3CH3 and BrCH2CH2Br all have one peak in their H NMR spectra because all of the protons in each molecule are equivalent. The compound below, 1,2- dibromo-2-methylpropane, has two peaks: one at 1.87 ppm (the equivalent CH3’s) and the other at 3.86 ppm (the CH2). 1.87 1.87 ppm CH 3 3.86 ppm Br Br CH3 1.87 ppm 3.86 10 9 8 7 6 5 4 3 2 1 0 2. How many 1H are in each environment? The relative intensities of the signals indicate the numbers of protons that are responsible for individual signals. The area under each peak is measured in the form of an integral line. -

Mechanism and Selectivity of Rhodium-Catalyzed 1:2 Coupling Of
Article pubs.acs.org/JACS Terms of Use Mechanism and Selectivity of Rhodium-Catalyzed 1:2 Coupling of Aldehydes and Allenes Genping Huang, Marcin Kalek, and Fahmi Himo* Department of Organic Chemistry, Arrhenius Laboratory, Stockholm University, SE-106 91 Stockholm, Sweden *S Supporting Information ABSTRACT: The rhodium-catalyzed highly regioselective 1:2 coupling of aldehydes and allenes was investigated by means of density functional theory calculations. Full free energy profiles were calculated, and several possible reaction pathways were evaluated. It is shown that the energetically most plausible catalytic cycle is initiated by oxidative coupling of the two allenes, which was found to be the rate-determining step of the overall reaction. Importantly, Rh−allyl complexes that are able to adopt both η3 and η1 configurations were identified as key intermediates present throughout the catalytic cycle with profound implications for the selectivity of the reaction. The calculations reproduced and rationalized the experimentally observed selectivities and provided an explanation for the remarkable alteration in the product distribution when the catalyst precursor is changed from [RhCl(nbd)]2 (nbd = norbornadiene) to complexes containing noncoordinating counterions ([Rh(cod)2X]; X = OTf, BF4,PF6; cod = 1,5-cyclooctadiene). It turns out that the overall selectivity of the reaction is controlled by a combination of the inherent selectivities of several of the elementary steps and that both the mechanism and the nature of the selectivity-determining steps change when the catalyst is changed. 1. INTRODUCTION comes from the laboratory of Murakami and consists of a 1:2 6 Rhodium(I) complexes are well-established catalysts for three- coupling of aldehydes and allenes (Scheme 2b). -
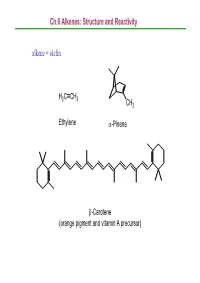
Ch.6 Alkenes: Structure and Reactivity Alkene = Olefin
Ch.6 Alkenes: Structure and Reactivity alkene = olefin H2CCH2 CH3 Ethylene α-Pinene β-Carotene (orange pigment and vitamin A precursor) Ch.6 Alkenes: Structure and Reactivity 6.1 Industrial Preparation and Use of Alkenes Compounds derived industrially from ethylene CH3CH2OH Ethanol CH3CHO Acetaldehyde CH3COOH Acetic acid HOCH2CH2OH Ethylene glycol ClCH2CH2Cl Ethylene dichloride H C=CHCl Vinyl chloride H2CCH2 2 O Ethylene oxide Ethylene (26 million tons / yr) O Vinyl acetate O Polyethylene Ch.6 Alkenes: Structure and Reactivity Compounds derived industrially from propylene OH Isopropyl alcohol H3CCH3 O Propylene oxide CH3 H3CCH CH2 Propylene Cumene (14 million tons / yr) CH3 CH3 Polypropylene Ch.6 Alkenes: Structure and Reactivity • Ethylene, propylene, and butene are synthesized industrially by thermal cracking of natural gas (C1-C4 alkanes) and straight-run gasoline (C4-C8 alkanes). 850-900oC CH (CH ) CH H + CH + H C=CH + CH CH=CH 3 2 n 3 steam 2 4 2 2 3 2 + CH3CH2CH=CH2 - the exact processes are complex; involve radical process H 900oC CH3CH2 CH2CH3 22H2CCH H2C=CH2 +H2 Ch.6 Alkenes: Structure and Reactivity • Thermal cracking is an example of a reaction whose energetics are dominated by entropy (∆So) rather than enthalpy (∆Ho) in the free-energy equation (∆Go = ∆Ho -T∆So) . ; C-C bond cleavage (positive ∆Ho) ; high T and increased number of molecules → larger T∆So Ch.6 Alkenes: Structure and Reactivity 6.2 Calculating Degree of Unsaturation unsaturated: formula of alkene CnH2n ; formula of alkane CnH2n+2 in general, each ring or double -

Ganic Compounds
6-1 SECTION 6 NOMENCLATURE AND STRUCTURE OF ORGANIC COMPOUNDS Many organic compounds have common names which have arisen historically, or have been given to them when the compound has been isolated from a natural product or first synthesised. As there are so many organic compounds chemists have developed rules for naming a compound systematically, so that it structure can be deduced from its name. This section introduces this systematic nomenclature, and the ways the structure of organic compounds can be depicted more simply than by full Lewis structures. The language is based on Latin, Greek and German in addition to English, so a classical education is beneficial for chemists! Greek and Latin prefixes play an important role in nomenclature: Greek Latin ½ hemi semi 1 mono uni 1½ sesqui 2 di bi 3 tri ter 4 tetra quadri 5 penta quinque 6 hexa sexi 7 hepta septi 8 octa octo 9 ennea nona 10 deca deci Organic compounds: Compounds containing the element carbon [e.g. methane, butanol]. (CO, CO2 and carbonates are classified as inorganic.) See page 1-4. Special characteristics of many organic compounds are chains or rings of carbon atoms bonded together, which provides the basis for naming, and the presence of many carbon- hydrogen bonds. The valency of carbon in organic compounds is 4. Hydrocarbons: Compounds containing only the elements C and H. Straight chain hydrocarbons are named according to the number of carbon atoms: CH4, methane; C2H6 or H3C-CH3, ethane; C3H8 or H3C-CH2-CH3, propane; C4H10 or H3C-CH2- CH2-CH3, butane; C5H12 or CH3CH2CH2CH2CH3, pentane; C6H14 or CH3(CH2)4CH3, hexane; C7H16, heptane; C8H18, octane; C9H20, nonane; C10H22, CH3(CH2)8CH3, decane. -

Total Synthesis of Malformin C, an Inhibitor of Bleomycin- Induced G2
J. Antibiot. 61(5): 297–302, 2008 THE JOURNAL OF ORIGINAL ARTICLE ANTIBIOTICS Total Synthesis of Malformin C, an Inhibitor of Bleomycin- Induced G2 Arrest Yasuhiro Kojima, Toshiaki Sunazuka, Kenichiro Nagai, Khachatur Julfakyan, Takashi Fukuda, Hiroshi Tomoda, Satoshi O¯ mura Received: April 14, 2008 / Accepted: May 3, 2008 © Japan Antibiotics Research Association Abstract Total synthesis of a fungal cyclic peptide, Jurkat cells with IC50 values of 480 nM and 0.9 nM, malformin C, recently rediscovered as a G2 checkpoint respectively [2]. Malformin A1 is a bicyclic pentapeptide inhibitor was completed. Our synthesis involved a containing one L-isoleucine, one D-leucine, one L-valine, convergent approach with respect to a linear pentapeptide, and two D-cysteines, while malformin C has L-leucine cyclization, and oxidative disulfide formation. instead of L-isoleucine. Interestingly, this slight structural difference of the side-chains has an influence on the Keywords total synthesis, malformin C, G2 checkpoint inhibitory activity. Consequently, malformin C is a inhibitor, anti-cancer reagent, cyclic peptide, disulfide promising candidate as a potentiator of anti-cancer agents. Although malformins are also known to show a variety of activities such as inducing root curvatures and Introduction malformations in plants, antibacterial activity and enhanced fibrinolytic activity [3ϳ6], the interesting bioactivity of Spontaneous and chemical damage to DNA induces signal malformin C, as well as its bicyclic structure with a transduction pathways called checkpoints, which delay cell disulfide-bond bridge, prompted us to study the synthesis cycle progression and repair of DNA [1]. DNA damage in of this class of cyclic peptides. Herein, we describe a normal human cells can be repaired in both the G1 and G2 synthesis of malformin C. -

Surface Chemistry Changes of Weathered HDPE/Wood-Flour
Polymer Degradation and Stability 86 (2004) 1–9 www.elsevier.com/locate/polydegstab Surface chemistry changes of weathered HDPE/wood-flour composites studied by XPS and FTIR spectroscopy* Nicole M. Starka,), Laurent M. Matuanab aU.S. Department of Agriculture, Forest Service, Forest Products Laboratory, One Gifford Pinchot Drive, Madison, WI 53726-2398, United States bDepartment of Forestry, Michigan State University, East Lansing, MI 48824-1222, United States Received 7 August 2003; received in revised form 3 November 2003; accepted 4 November 2003 Abstract The use of wood-derived fillers by the thermoplastic industry has been growing, fueled in part by the use of wood-fiber– thermoplastic composites by the construction industry. As a result, the durability of wood-fiber–thermoplastic composites after ultraviolet exposure has become a concern. Samples of 100% high-density polyethylene (HDPE) and HDPE filled with 50% wood- flour (WF) were weathered in a xenon arc-type accelerated weathering apparatus for 2000 h. Changes in surface chemistry were studied using spectroscopic techniques. X-ray photoelectron spectroscopy (XPS) was used to verify the occurrence of surface oxidation. Fourier transform infrared (FTIR) spectroscopy was used to monitor the development of degradation products, such as carbonyl groups and vinyl groups, and to determine changes in HDPE crystallinity. The results indicate that surface oxidation occurred immediately after exposure for both the neat HDPE and WF/HDPE composites; the surface of the WF/HDPE composites was oxidized to a greater extent than that of the neat HDPE. This suggests that the addition of WF to the HDPE matrix results in more weather-related damage. -

Novel Poly(Vinyl Alcohol)-Based Column Coating for Capillary Electrophoresis of Proteins
Biochemical Engineering Journal 53 (2010) 137–142 Contents lists available at ScienceDirect Biochemical Engineering Journal journal homepage: www.elsevier.com/locate/bej Novel poly(vinyl alcohol)-based column coating for capillary electrophoresis of proteins Liang Xu, Xiao-Yan Dong, Yan Sun ∗ Department of Biochemical Engineering and Key Laboratory of Systems Bioengineering of the Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China article info abstract Article history: A novel and simple method for the preparation of chemically bonded poly(vinyl alcohol) (PVA) coat- Received 30 June 2010 ing to silica capillary inner wall was developed, and the PVA-coated capillary columns were employed Received in revised form for capillary electrophoresis (CE). The coating procedure included pretreatment of the capillary inner 30 September 2010 wall, silanization, aldehyde group functionalization and PVA immobilization. Electroosmotic flow of the Accepted 6 October 2010 coated capillary was almost suppressed over a wide pH range (pH 3–10). High-efficiency separations of cationic proteins (including cytochrome c, lysozyme, ␣-chymotrypsinogen A) at pH 3.0–5.0 and of anionic proteins (including myoglobin and trypsin inhibitor) at pH 10.0 were achieved with the PVA-coated cap- Keywords: Protein illary. Moreover, a “dual-opposite-injection” approach was adopted for simultaneous separations of both Separation cationic and anionic proteins at neutral pH with the prepared column. In this CE mode, positively charged Bioprocess Monitoring proteins migrated from one end of the column to the detector while negatively charged proteins from Adsorption the other end to the detection window. Good run-to-run repeatability was obtained in all of the protein Capillary electrophoresis CE separations performed in this work. -

TOX-48: Allyl Acetate (CASRN 591-87-7), Allyl Alcohol (CASRN
National Toxicology Program Toxicity Report Series Number 48 NTP Technical Report on the Comparative Toxicity Studies of Allyl Acetate, Allyl Alcohol, and Acrolein (CAS Nos. 591-87-7, 107-18-6, and 107-02-8) Administered by Gavage to F344/N Rats and B6C3F1 Mice July 2006 National Institutes of Health Public Health Service U.S. Department of Health and Human Services FOREWORD The National Toxicology Program (NTP) is an interagency program within the Public Health Service (PHS) of the Department of Health and Human Services (HHS) and is headquartered at the National Institute of Environmental Health Sciences of the National Institutes of Health (NIEHS/NIH). Three agencies contribute resources to the program: NIEHS/NIH, the National Institute for Occupational Safety and Health of the Centers for Disease Control and Prevention (NIOSH/CDC), and the National Center for Toxicological Research of the Food and Drug Administration (NCTR/FDA). Established in 1978, the NTP is charged with coordinating toxicological testing activities, strengthening the science base in toxicology, developing and validating improved testing methods, and providing information about potentially toxic substances to health regulatory and research agencies, scientific and medical communities, and the public. The Toxicity Study Report series began in 1991. The studies described in the Toxicity Study Report series are designed and conducted to characterize and evaluate the toxicologic potential of selected substances in laboratory animals (usually two species, rats and mice). Substances selected for NTP toxicity studies are chosen primarily on the basis of human exposure, level of production, and chemical structure. The interpretive conclusions presented in the Toxicity Study Reports are based only on the results of these NTP studies. -

Alkynes: Molecular and Structural Formulas
Alkynes: Molecular and Structural Formulas The alkynes comprise a series of carbon- and hydrogen-based compounds that contain at least one triple bond. This group of compounds is a homologous series with the general molecular formula of C n H2 n--2 , where n equals any integer greater than one. The simplest alkyne, ethyne (also known as acetylene), has two carbon atoms and the molecular formula of C2H2. The structural formula for ethyne is In longer alkyne chains, the additional carbon atoms are attached to each other by single covalent bonds. Each carbon atom is also attached to sufficient hydrogen atoms to produce a total of four single covalent bonds about itself. In alkynes of four or more carbon atoms, the triple bond can be located in different positions along the chain, leading to the formation of structural isomers. For example, the alkyne of molecular formula C4H6 has two isomers, Although alkynes possess restricted rotation due to the triple bond, they do not have stereoisomers like the alkenes because the bonding in a carbon-carbon triple bond is sp hybridized. In sp hybridization, the maximum separation between the hybridized orbitals is 180°, so the molecule is linear. Thus, the substituents on triple-bonded carbons are positioned in a straight line, and stereoisomers are impossible Alkynes: Nomenclature Although some common alkyne names, such as acetylene, are still found in many textbooks, the International Union of Pure and Applied Chemistry (IUPAC) nomenclature is required for journal articles. The rules for alkynes in this system are identical with those for alkenes, except for the ending. -

New Reactions of Ring Strained Allyl Silanes
Western Washington University Western CEDAR WWU Graduate School Collection WWU Graduate and Undergraduate Scholarship Summer 2018 New Reactions of Ring Strained Allyl Silanes Elizabeth J. (Elizabeth Jane) Cummins Western Washington University, [email protected] Follow this and additional works at: https://cedar.wwu.edu/wwuet Part of the Chemistry Commons Recommended Citation Cummins, Elizabeth J. (Elizabeth Jane), "New Reactions of Ring Strained Allyl Silanes" (2018). WWU Graduate School Collection. 749. https://cedar.wwu.edu/wwuet/749 This Masters Thesis is brought to you for free and open access by the WWU Graduate and Undergraduate Scholarship at Western CEDAR. It has been accepted for inclusion in WWU Graduate School Collection by an authorized administrator of Western CEDAR. For more information, please contact [email protected]. New Reactions of Ring Strained Allyl Silanes By Elizabeth Jane Cummins Accepted in Partial Completion of the Requirements for the Degree Master of Science ADVISORY COMMITTEE Dr. Gregory O’Neil, Chair Dr. James Vyvyan Dr. John Antos Western Washington University College of Science and Engineering Dr. Gautam Pillay, Dean Master’s Thesis In presenting this thesis in partial fulfillment of the requirements for a master’s degree at Western Washington University, I grant to Western Washington University the non-exclusive royalty-free right to archive, reproduce, distribute, and display the thesis in any and all forms, including electronic format, via any digital library mechanisms maintained by WWU. I represent and warrant this is my original work, and does not infringe or violate any rights of others. I warrant that I have obtained written permissions from the owner of any third party copyrighted material included in these files.