Synthesis of 3D-PEPS According to 2 Related Pathways
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mechanical Properties for Polyester Resin Reinforce with Fe Weave Wire
International Journal of Application or Innovation in Engineering & Management (IJAIEM) Web Site: www.ijaiem.org Email: [email protected] Volume 3, Issue 7, July 2014 ISSN 2319 - 4847 Mechanical Properties for Polyester resin Reinforce with Fe Weave Wire Alaa A. Abdul-Hamead 1, Thekra Kasim2, and Awattiff A.Mohammed 3 1 Materials Eng. Department University of Technology 2Department of Physics, College of Science, University of Baghdad, Baghdad, Iraq 3Department of Physics, College of Science, University of Baghdad, Baghdad, Iraq ABSTRACT In the present work we prepared and study a polymer composite using from and polyester polymer with iron weave wire ratios (5,10,15,20%) .At first was studied and examined with chemical composition analyzing, then some of physical and mechanical properties of the composite were studied stress –strain , impact strength, fracture toughness , hardness and thermal conductivity. Results show an improvement in these mechanical properties after reinforcement by metals the value of mechanical properties will increase with increasing percentage of reinforcement. Keywords: polymer composite, polyester , Fe weave wire , impact strength, fracture toughness, hardness & thermal conductivity. 1. INTRODUCTION Composite materials were created as a result of an intensive search for materials which offer high reinforcement levels, with good mechanical properties and light weight.[1] Polymer composites can be classified as :Macro-composites ,Micro- composites and Nanocomposites according to fillers size[2,3]. Unsaturated polymers (UPE) resin is used for a wide variety of industrial and consumer applications. This consumption can be split into two major categories of applications: reinforced and nonreinforced. In reinforced applications, resin and reinforcement, such as fiberglass, are used together to produce a composite with improved physical properties. -

Proton Nmr Spectroscopy
1H NMR Spectroscopy (#1c) The technique of 1H NMR spectroscopy is central to organic chemistry and other fields involving analysis of organic chemicals, such as forensics and environmental science. It is based on the same principle as magnetic resonance imaging (MRI). This laboratory exercise reviews the principles of interpreting 1H NMR spectra that you should be learning right now in Chemistry 302. There are four questions you should ask when you are trying to interpret an NMR spectrum. Each of these will be discussed in detail. The Four Questions to Ask While Interpreting Spectra 1. How many different environments are there? The number of peaks or resonances (signals) in the spectrum indicates the number of nonequivalent protons in a molecule. Chemically equivalent protons (magnetically equivalent protons) give the same signal in the NMR whereas nonequivalent protons give different signals. 1 For example, the compounds CH3CH3 and BrCH2CH2Br all have one peak in their H NMR spectra because all of the protons in each molecule are equivalent. The compound below, 1,2- dibromo-2-methylpropane, has two peaks: one at 1.87 ppm (the equivalent CH3’s) and the other at 3.86 ppm (the CH2). 1.87 1.87 ppm CH 3 3.86 ppm Br Br CH3 1.87 ppm 3.86 10 9 8 7 6 5 4 3 2 1 0 2. How many 1H are in each environment? The relative intensities of the signals indicate the numbers of protons that are responsible for individual signals. The area under each peak is measured in the form of an integral line. -

Poly (Ethylene Terephthalate) Recycling for High Value Added Textiles Sang Ho Park and Seong Hun Kim*
Park and Kim Fashion and Textiles 2014, 1:1 http://link.springer.com/article/10.1186/s40691-014-0001-x REVIEW Open Access Poly (ethylene terephthalate) recycling for high value added textiles Sang Ho Park and Seong Hun Kim* * Correspondence: [email protected] Abstract Department of Organic anc Nano Engineering, College of This study reviews the problems in the use and disposal of poly (ethylene Engineering, Hanyang University, 17 terephthalate) (PET) and includes the concise background of virgin and recycled PET Haengdang-dong, Sungdong-gu, as well as their possible applications. The current state of knowledge with respect to Seoul 133-791, Korea PET recycling method is presented. Recycling of PET is the most desirable method for waste management, providing an opportunity for reductions in oil usage, carbon dioxide emissions and PET waste requiring disposal because of its non-degradability. Advanced technologies and systems for reducing contamination, mechanical and chemical recycling, and their applications are discussed, and the possibility of diverting the majority of PET waste from landfills or incineration to recycling is suggested. Keywords: Polyethylene terephthalate; Mechanical recycle; Chemical recycle Introduction Poly (ethylene terephthalate) (PET), commonly referred to as ‘polyester’ in the textile industry, is considered to be one of the most important thermoplastic polyesters (Incornato et al. 2000). It is widely used for various applications such as bottles, fibers, moldings, and sheets because of its excellent tensile and impact strength, clarity, pro- cessability, chemical resistance, and thermal stability (Pawlak et al. 2000; Kong and Hay 2003; Avila-Orta et al. 2003). The PET fiber patented originally by DuPont (DuPont, 1997) dominates over 50% of the world synthetic fiber market. -
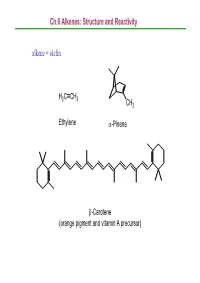
Ch.6 Alkenes: Structure and Reactivity Alkene = Olefin
Ch.6 Alkenes: Structure and Reactivity alkene = olefin H2CCH2 CH3 Ethylene α-Pinene β-Carotene (orange pigment and vitamin A precursor) Ch.6 Alkenes: Structure and Reactivity 6.1 Industrial Preparation and Use of Alkenes Compounds derived industrially from ethylene CH3CH2OH Ethanol CH3CHO Acetaldehyde CH3COOH Acetic acid HOCH2CH2OH Ethylene glycol ClCH2CH2Cl Ethylene dichloride H C=CHCl Vinyl chloride H2CCH2 2 O Ethylene oxide Ethylene (26 million tons / yr) O Vinyl acetate O Polyethylene Ch.6 Alkenes: Structure and Reactivity Compounds derived industrially from propylene OH Isopropyl alcohol H3CCH3 O Propylene oxide CH3 H3CCH CH2 Propylene Cumene (14 million tons / yr) CH3 CH3 Polypropylene Ch.6 Alkenes: Structure and Reactivity • Ethylene, propylene, and butene are synthesized industrially by thermal cracking of natural gas (C1-C4 alkanes) and straight-run gasoline (C4-C8 alkanes). 850-900oC CH (CH ) CH H + CH + H C=CH + CH CH=CH 3 2 n 3 steam 2 4 2 2 3 2 + CH3CH2CH=CH2 - the exact processes are complex; involve radical process H 900oC CH3CH2 CH2CH3 22H2CCH H2C=CH2 +H2 Ch.6 Alkenes: Structure and Reactivity • Thermal cracking is an example of a reaction whose energetics are dominated by entropy (∆So) rather than enthalpy (∆Ho) in the free-energy equation (∆Go = ∆Ho -T∆So) . ; C-C bond cleavage (positive ∆Ho) ; high T and increased number of molecules → larger T∆So Ch.6 Alkenes: Structure and Reactivity 6.2 Calculating Degree of Unsaturation unsaturated: formula of alkene CnH2n ; formula of alkane CnH2n+2 in general, each ring or double -

Role of Thermosetting Polymer in Structural Composite
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Ivy Union Publishing (E-Journals) American Journal of Polymer Science & Engineering Kausar A. American Journal of Polymer Sciencehttp://www.ivyunion.org/index.php/ajpse/ & Engineering 2017, 5:1-12 Page 1 of 12 Review Article Role of Thermosetting Polymer in Structural Composite Ayesha Kausar1 1 Nanoscience and Technology Department, National Center For Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan Abstract Thermosetting resins are network forming polymers with highly crosslinked structure. In this review article, thermoset of epoxy, unsaturated polyester resin, phenolic, melamine, and polyurethane resin have been conversed. Thermosets usually have outstanding tensile strength, impact strength, and glass transition temperature (Tg). Epoxy is the most widely explored class of thermosetting resins. Owing to high stiffness and strength, chemical resistance, good dielectric behavior, corrosion resistance, low shrinkage during curing, and good thermal features, epoxy form the most important class of thermosetting resins for several engineering applications. Here, essential features of imperative thermosetting resins have been discussed such as mechanical, thermal, and non-flammability. At the end, employment of thermosetting resins in technical applications like sporting goods, adhesives, printed circuit board, and aerospace have been included. Keywords: Thermoset; epoxy; mechanical; non-flammability; application Received : November 14, 2016; Accepted: January 8, 2017; Published: January 16, 2017 Competing Interests: The authors have declared that no competing interests exist. Copyright: 2017 Kausar A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. -

Ganic Compounds
6-1 SECTION 6 NOMENCLATURE AND STRUCTURE OF ORGANIC COMPOUNDS Many organic compounds have common names which have arisen historically, or have been given to them when the compound has been isolated from a natural product or first synthesised. As there are so many organic compounds chemists have developed rules for naming a compound systematically, so that it structure can be deduced from its name. This section introduces this systematic nomenclature, and the ways the structure of organic compounds can be depicted more simply than by full Lewis structures. The language is based on Latin, Greek and German in addition to English, so a classical education is beneficial for chemists! Greek and Latin prefixes play an important role in nomenclature: Greek Latin ½ hemi semi 1 mono uni 1½ sesqui 2 di bi 3 tri ter 4 tetra quadri 5 penta quinque 6 hexa sexi 7 hepta septi 8 octa octo 9 ennea nona 10 deca deci Organic compounds: Compounds containing the element carbon [e.g. methane, butanol]. (CO, CO2 and carbonates are classified as inorganic.) See page 1-4. Special characteristics of many organic compounds are chains or rings of carbon atoms bonded together, which provides the basis for naming, and the presence of many carbon- hydrogen bonds. The valency of carbon in organic compounds is 4. Hydrocarbons: Compounds containing only the elements C and H. Straight chain hydrocarbons are named according to the number of carbon atoms: CH4, methane; C2H6 or H3C-CH3, ethane; C3H8 or H3C-CH2-CH3, propane; C4H10 or H3C-CH2- CH2-CH3, butane; C5H12 or CH3CH2CH2CH2CH3, pentane; C6H14 or CH3(CH2)4CH3, hexane; C7H16, heptane; C8H18, octane; C9H20, nonane; C10H22, CH3(CH2)8CH3, decane. -

Surface Chemistry Changes of Weathered HDPE/Wood-Flour
Polymer Degradation and Stability 86 (2004) 1–9 www.elsevier.com/locate/polydegstab Surface chemistry changes of weathered HDPE/wood-flour composites studied by XPS and FTIR spectroscopy* Nicole M. Starka,), Laurent M. Matuanab aU.S. Department of Agriculture, Forest Service, Forest Products Laboratory, One Gifford Pinchot Drive, Madison, WI 53726-2398, United States bDepartment of Forestry, Michigan State University, East Lansing, MI 48824-1222, United States Received 7 August 2003; received in revised form 3 November 2003; accepted 4 November 2003 Abstract The use of wood-derived fillers by the thermoplastic industry has been growing, fueled in part by the use of wood-fiber– thermoplastic composites by the construction industry. As a result, the durability of wood-fiber–thermoplastic composites after ultraviolet exposure has become a concern. Samples of 100% high-density polyethylene (HDPE) and HDPE filled with 50% wood- flour (WF) were weathered in a xenon arc-type accelerated weathering apparatus for 2000 h. Changes in surface chemistry were studied using spectroscopic techniques. X-ray photoelectron spectroscopy (XPS) was used to verify the occurrence of surface oxidation. Fourier transform infrared (FTIR) spectroscopy was used to monitor the development of degradation products, such as carbonyl groups and vinyl groups, and to determine changes in HDPE crystallinity. The results indicate that surface oxidation occurred immediately after exposure for both the neat HDPE and WF/HDPE composites; the surface of the WF/HDPE composites was oxidized to a greater extent than that of the neat HDPE. This suggests that the addition of WF to the HDPE matrix results in more weather-related damage. -

4.4 Polyester Resin Plastic Products Fabrication 4.4.1 General
4.4 Polyester Resin Plastic Products Fabrication 4.4.1 General Description1-2 A growing number of products are fabricated from liquid polyester resin reinforced with glass fibers and extended with various inorganic filler materials such as calcium carbonate, talc, mica, or small glass spheres. These composite materials are often referred to as fiberglass-reinforced plastic (FRP), or simply "fiberglass". The Society Of The Plastics industry designates these materials as "reinforced plastics/composites" (RP/C). Also, advanced reinforced plastics products are now formulated with fibers other than glass, such as carbon, aramid, and aramid/carbon hybrids. In some processes, resin products are fabricated without fibers. One major product using resins with fillers but no reinforcing fibers is the synthetic marble used in manufacturing bathroom countertops, sinks, and related items. Other applications of nonreinforced resin plastics include automobile body filler, bowling balls, and coatings. Fiber-reinforced plastics products have a wide range of application in industry, transportation, home, and recreation. Industrial uses include storage tanks, skylights, electrical equipment, ducting, pipes, machine components, and corrosion resistant structural and process equipment. In transportation, automobile and aircraft applications are increasing rapidly. Home and recreational items include bathroom tubs and showers, boats (building and repair), surfboards and skis, helmets, swimming pools and hot tubs, and a variety of sporting goods. The thermosetting polyester resins considered here are complex polymers resulting from the cross-linking reaction of a liquid unsaturated polyester with a vinyl type monomer, list often styrene. The unsaturated polyester is formed from the condensation reaction of an unsaturated dibasic acid or anhydride, a saturated dibasic acid or anhydride, and a polyfunctional alcohol. -

Castin'craft Casting Resin Basics, Instructions and Tips
CASTIN’CRAFT CASTING RESIN BASICS, INSTRUCTIONS AND TIPS BASICS OF RESIN CASTING (cured) stage in 1 to 24 hours. The length of this cycle will vary Resin casting is an exciting and fun craft that allows you to greatly depending on the four factors mentioned previously. The embed or encase almost any object in crystal-clear plastic. The period of time between the addition of the catalyst and the gel basic materials needed to get started in resin crafting are easy to stage is called the 'working time' or 'pot life' of the resin. find and relatively inexpensive. Generally this is about 15 to 20 minutes. Do not catalyze more You'll need: resin than you can pour during the 'working time' since catalyzed 1. Casting resin and catalyst resin cannot be poured once it has gelled. Do not pour catalyzed 2. Disposable graduated paper mixing cups resin back into your casting resin can. Catalyst should be stored 3. Wooden stir sticks at room temperature, out of sunlight and out of reach of children. 4. A mold Shelf life is indefinite as long as stored properly. 5. Objects you wish to embed EMBEDMENTS Color dyes and pigments are optional and can be used to create a Here are some suggested objects that can be suspended or variety of special effects and backgrounds. Resin crafting encased in casting resin: • Crushed glass • Coins • Fabric* • supplies are available at hobby, craft and plastics supply stores. Flowers - dried or pressed • Glass jewels or marbles • Glitter • Insects or biological specimens • Jewelry findings • Leaves - Understanding the basics of how casting resin can be changed dried or pressed • Mechanical parts, nuts bolts etc. -

Novel Poly(Vinyl Alcohol)-Based Column Coating for Capillary Electrophoresis of Proteins
Biochemical Engineering Journal 53 (2010) 137–142 Contents lists available at ScienceDirect Biochemical Engineering Journal journal homepage: www.elsevier.com/locate/bej Novel poly(vinyl alcohol)-based column coating for capillary electrophoresis of proteins Liang Xu, Xiao-Yan Dong, Yan Sun ∗ Department of Biochemical Engineering and Key Laboratory of Systems Bioengineering of the Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China article info abstract Article history: A novel and simple method for the preparation of chemically bonded poly(vinyl alcohol) (PVA) coat- Received 30 June 2010 ing to silica capillary inner wall was developed, and the PVA-coated capillary columns were employed Received in revised form for capillary electrophoresis (CE). The coating procedure included pretreatment of the capillary inner 30 September 2010 wall, silanization, aldehyde group functionalization and PVA immobilization. Electroosmotic flow of the Accepted 6 October 2010 coated capillary was almost suppressed over a wide pH range (pH 3–10). High-efficiency separations of cationic proteins (including cytochrome c, lysozyme, ␣-chymotrypsinogen A) at pH 3.0–5.0 and of anionic proteins (including myoglobin and trypsin inhibitor) at pH 10.0 were achieved with the PVA-coated cap- Keywords: Protein illary. Moreover, a “dual-opposite-injection” approach was adopted for simultaneous separations of both Separation cationic and anionic proteins at neutral pH with the prepared column. In this CE mode, positively charged Bioprocess Monitoring proteins migrated from one end of the column to the detector while negatively charged proteins from Adsorption the other end to the detection window. Good run-to-run repeatability was obtained in all of the protein Capillary electrophoresis CE separations performed in this work. -

Materials for Polymer Composites
Module 2 - Materials For Polymer Composites Module 2 - Materials for Polymer Composites Introduction Major constituents in a fiber-reinforced composite material are the reinforcing fibers and a matrix, which acts as a binder for the fibers. Other constituents that may also be found are coupling agents, coatings, and fillers. Coupling agents and coatings are applied on the fibers to improve their wetting with the matrix as well as to promote bonding across the fiber–matrix interface. Both in turn promote a better load transfer between the fibers and the matrix. Fillers are used with some polymeric matrices primarily to reduce cost and improve their dimensional stability. Manufacturing of a composite structure starts with the incorporation of a large number of fibers into a thin layer of matrix to form a lamina (ply). The thickness of a lamina is usually in the range of 0.1–1 mm. If continuous (long) fibers are used in making the lamina, they may be arranged either in a unidirectional orientation (i.e., all fibers in one direction, Figure 2.1a), in a bidirectional orientation (i.e., fibers in two directions, usually normal to each other, Figure 2.1b), or in a multidirectional orientation (i.e., fibers in more than two directions, Figure 2.1c). The bi- or multidirectional orientation of fibers is obtained by weaving or other processes used in the textile industry. For a lamina containing unidirectional fibers, the composite material has the highest strength and modulus in the longitudinal direction of the fibers. However, in the transverse direction, its strength and modulus are very low. -

Alkynes: Molecular and Structural Formulas
Alkynes: Molecular and Structural Formulas The alkynes comprise a series of carbon- and hydrogen-based compounds that contain at least one triple bond. This group of compounds is a homologous series with the general molecular formula of C n H2 n--2 , where n equals any integer greater than one. The simplest alkyne, ethyne (also known as acetylene), has two carbon atoms and the molecular formula of C2H2. The structural formula for ethyne is In longer alkyne chains, the additional carbon atoms are attached to each other by single covalent bonds. Each carbon atom is also attached to sufficient hydrogen atoms to produce a total of four single covalent bonds about itself. In alkynes of four or more carbon atoms, the triple bond can be located in different positions along the chain, leading to the formation of structural isomers. For example, the alkyne of molecular formula C4H6 has two isomers, Although alkynes possess restricted rotation due to the triple bond, they do not have stereoisomers like the alkenes because the bonding in a carbon-carbon triple bond is sp hybridized. In sp hybridization, the maximum separation between the hybridized orbitals is 180°, so the molecule is linear. Thus, the substituents on triple-bonded carbons are positioned in a straight line, and stereoisomers are impossible Alkynes: Nomenclature Although some common alkyne names, such as acetylene, are still found in many textbooks, the International Union of Pure and Applied Chemistry (IUPAC) nomenclature is required for journal articles. The rules for alkynes in this system are identical with those for alkenes, except for the ending.